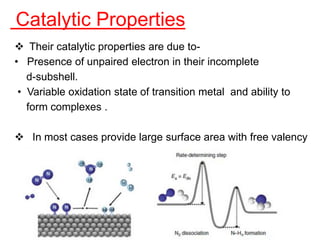
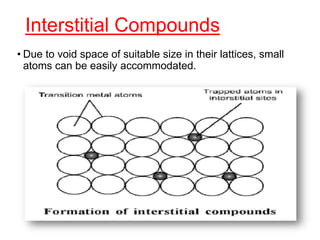
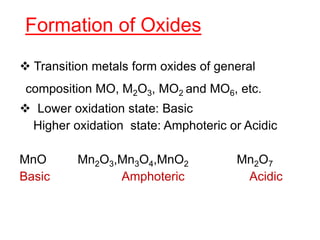
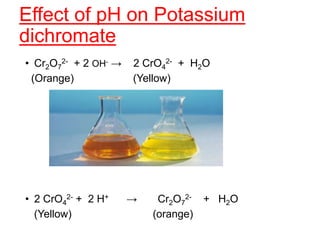
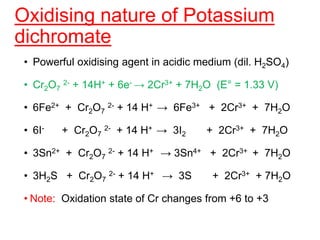
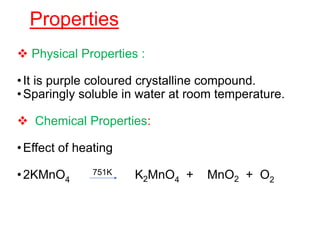
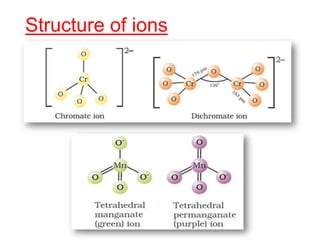
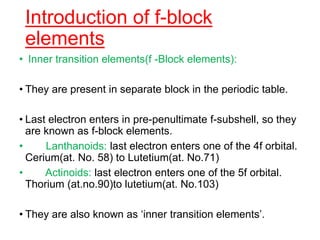
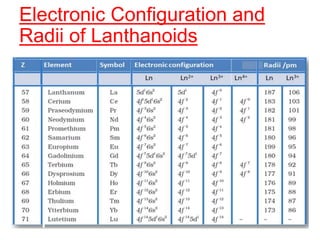
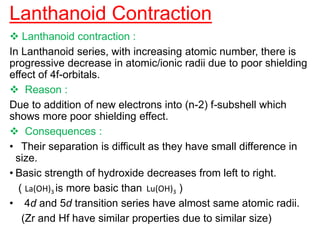
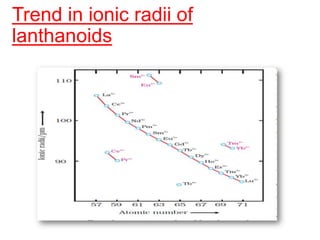
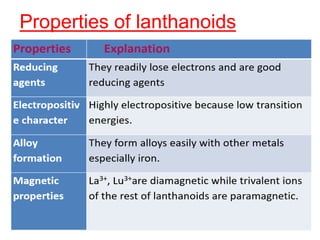
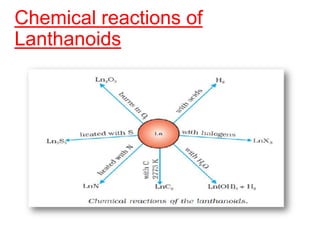
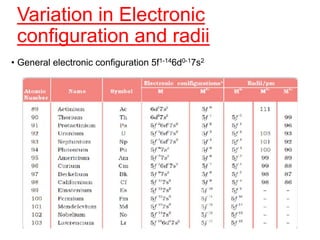
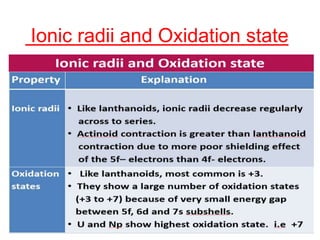
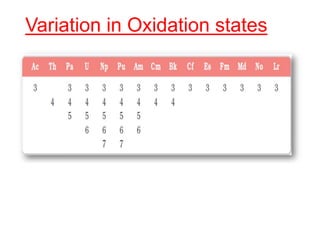
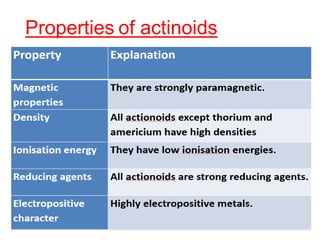
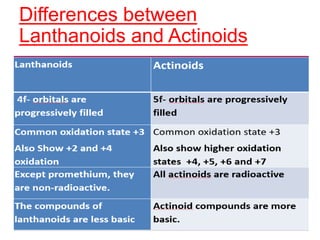
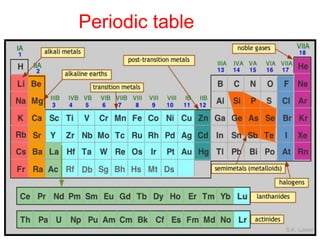
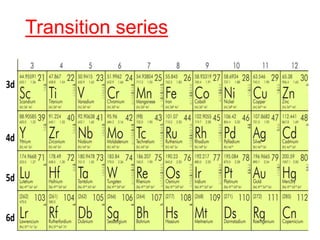
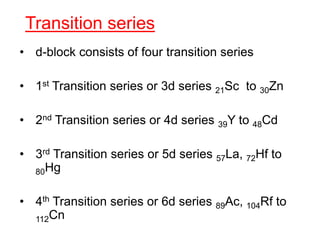
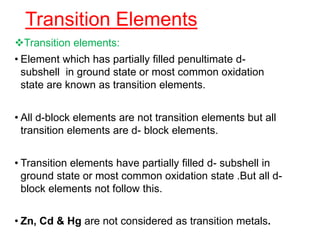
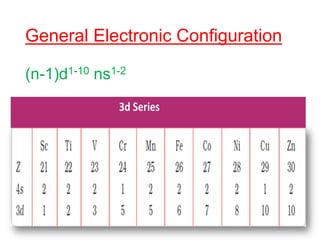
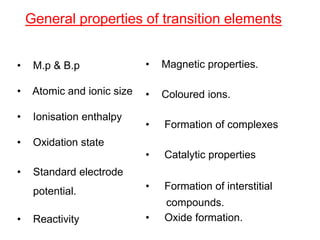
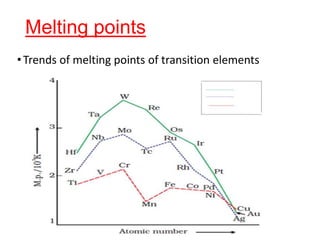
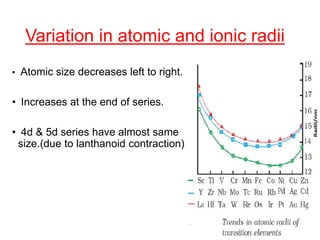
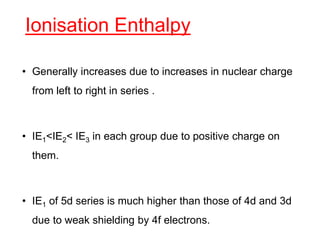
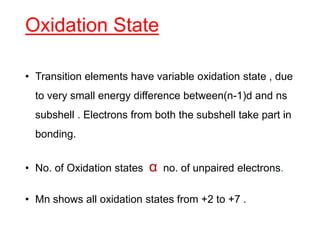
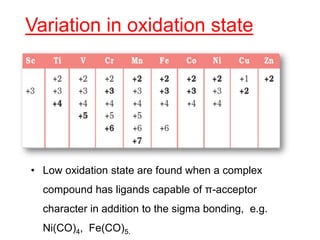
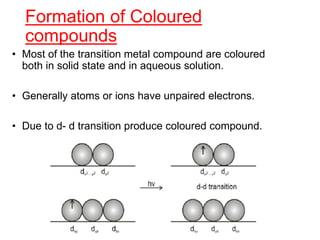
The document discusses the d-block and f-block elements. It describes the transition elements as having electrons in the d-orbital. The d-block elements are divided into four transition series based on their electronic configuration. Key properties of transition elements include variable oxidation states and catalytic and magnetic properties. The f-block elements are the lanthanides and actinides which have electrons in the 4f and 5f orbitals respectively. They exhibit lanthanide contraction which decreases their atomic radii across the period.




![Complex Formation
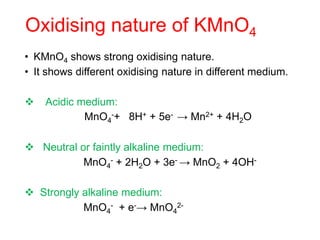
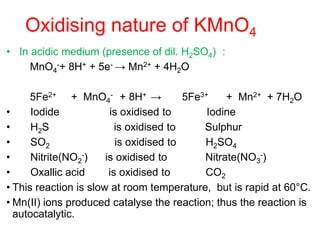
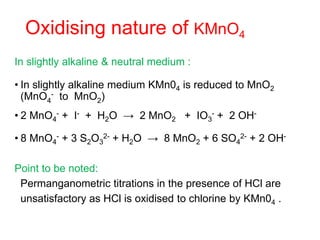
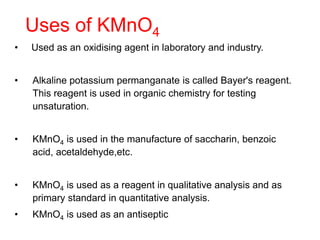
• The tendency to form complex compounds is due to-
• Small size of the ion
• High charge on the transition metal ion.
• The availability of d orbitals for accommodating
electrons donated by the ligands.
• Cu2+ (aq) + 4 NH3 (aq) [Cu(NH3)4]2+ (aq)
(blue) (deep blue)
• AgCl (s) + 2 NH3 (aq) → [Ag (NH3)2]Cl (aq)
(white ppt) (Colourless )](https://image.slidesharecdn.com/dandfblockelements-221026075640-b745b615/85/d-and-f-block-elements-pptx-28-320.jpg)