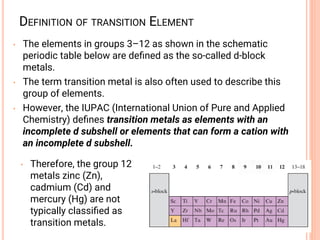
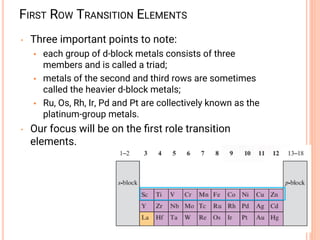
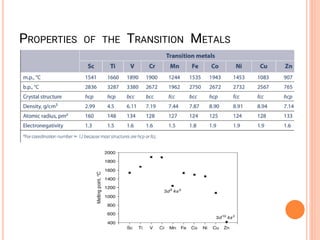
This document provides a summary of key concepts regarding transition elements covered in an inorganic chemistry lecture note. It discusses the definition of transition elements, their electronic configurations, atomic radii, ionization potentials, variable oxidation states, and ability to form metal complexes. It also introduces coordination chemistry concepts like metal complexes, ligands, and bonding theories. Properties of octahedral and tetrahedral complexes are examined along with nomenclature of coordination compounds.


![ELECTRONIC
CONFIGURATION
• The observed ground state electronic
configurations of the first row d-block metal
atoms almost correspond to the progressive filling
of the 3d, atomic orbitals respectively.
• However, there are minor deviations from this
pattern, e.g. in the first row, the ground state
of chromium (atomic number 24) is [Ar]4s13d5 rather
than [Ar]4s23d4.
• Same thing is witnessed in Cu (atomic number 29)
is [Ar]4s13d10 rather than [Ar]4s23d9.
• A reason for this deviation is fairly complicated
but related to the energy difference between the
3d and 4s atomic orbitals and the stability of 3d
when it is half or fully filled.](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-6-320.jpg)
![IONIC FORM
• d-Block metals can show several
oxidation states as their valence
electrons can be present in more than
one atomic orbital.
• M2+ and M3+ ions of the first-row d-
block metals follow the general
formula [Ar]3dn which implies that the
electron loss is at the 4s orbital.
• Thus, the comparative chemistry of
these metals is largely concerned with
the consequences of the successive
filling of the 3d orbitals.](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-7-320.jpg)


![QUESTION
1. The electronic configuration of chromium is
(a) [Ar] 3d44s2
(b) [Ar] 3d5 4s1
(c) [Ar] 3d6 4s0
(d) [Ar] 3d34s2
2. Give the electronic configuration of the following atoms
and ions:
(i) V (ii) Cu (iii) Zn (iv) Co2+ (v) Cr3+ (vi) Fe3+](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-10-320.jpg)


![QUESTION
1.The melting/boiling point of first row
transition metals across the period
(a) Decrease across the period
(b) Decrease up to d4, then increase
(c) Remains constant
(d) Increase up to d4, then decrease
2. Which of these ions is not coloured?
Explain why?
(A) [Co(SCN)4]2-
(B) [Fe(H2O)5SCN]2+
(C) [Al(OH)4]2-
(D) Ni(DMG)2](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-14-320.jpg)


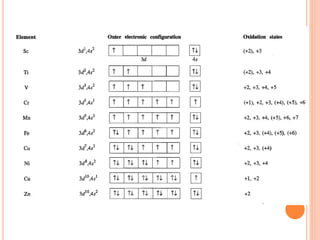
![VARIABLE OXIDATION STATES
• Oxidation states of the d-block metals; the most
important and stable states are marked in blue.
• Tabulation of zero oxidation states refers to their
appearance in compounds of the metal.
• In organometallic compounds, oxidation states of less
than zero are encountered .
• An oxidation state enclosed in [ ] is rare.](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-19-320.jpg)







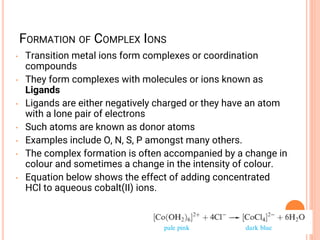

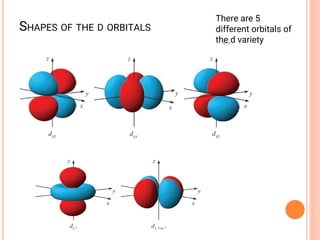
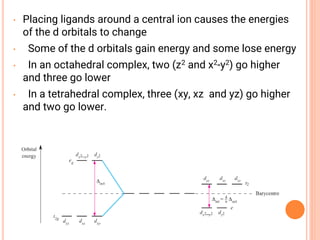
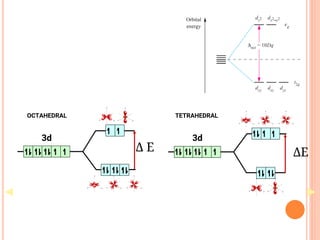

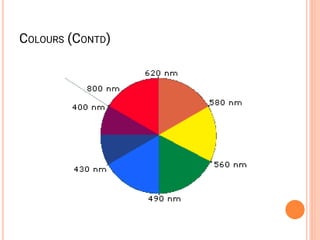
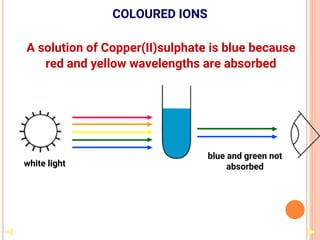



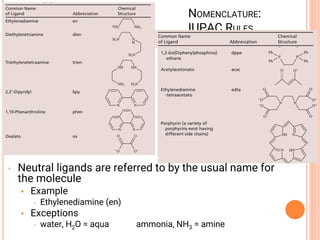
![COORDINATION CHEMISTRY
• A coordination compound is formed by
joining independent molecules or ions (known
collectively as the ligands) to a central atom
or transition metal ion using coordinate
covalent bonds.
• Ligands are atoms, or ions, which possess
lone pairs of electrons.
• Ligands form co-ordinate bonds to the central
ion by donating a lone pair into vacant
orbitals on the central species
[Co(NH3)5Cl]Cl2
[Fe(en)2(NO2)2]2SO4](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-44-320.jpg)


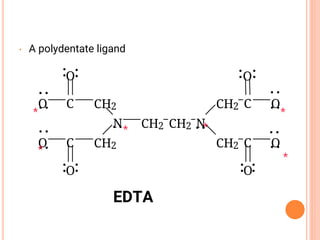
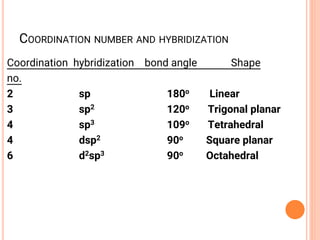
![CO-ORDINATION NUMBER & SHAPE
the shape of a complex is governed by the number of ligands around the central ion
the co-ordination number gives the number of ligands around the central ion
a change of ligand can affect the co-ordination number
Co-ordination No. Shape Example(s)
6 Octahedral [Cu(H2O)6]2+
4 Tetrahedral [CuCl4]2-
Square planar [Pt(NH3)2Cl2]
2 Linear [Ag(NH ) ]+](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-48-320.jpg)
![Common Geometries of
Complexes
Linear
Coordination Number Geometry
2
Example: [Ag(NH3)2]+](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-49-320.jpg)
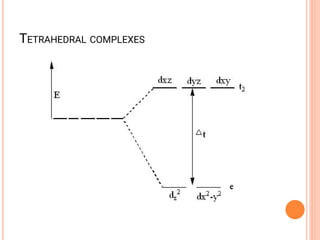
![Common Geometries of Complexes
Coordination Number Geometry
4
tetrahedral
square planar
Example: [Ni(CN)4]2-
Examples: [Zn(NH3)4]2+,
[FeCl4]-
(characteristic of metal ions with 8 d e-’s)](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-50-320.jpg)
![Common Geometries of Complexes
Coordination Number Geometry
6
octahedral
Examples: [Co(CN)6]3-,
[Fe(en)3]3+](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-51-320.jpg)

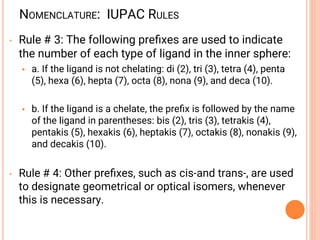
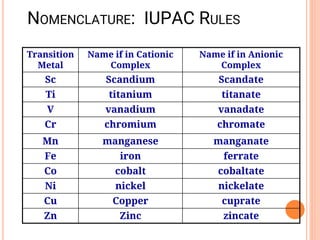
![NOMENCLATURE OF COORDINATION COMPOUNDS:
IUPAC RULES
➢ Rule # 1: The cation is listed first, then a space, followed by the
anion.
➢ For example the cation in this is named
- [Co(NH3)5Cl]Cl2
before the anion (chloride), which is named last
➢ Rule # 2: When naming the cation complex:
• Any ligands that are part of the inner coordination sphere are listed
first, followed immediately by the name of the metal with its
oxidation state in parentheses.
• Ligands are named in alphabetical order (When writing formular of a
complex, anions are placed before neutral molecules)
• Metal atom/ion is named last
• oxidation state given in Roman numerals follows in parentheses
• Use no spaces in complex name](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-55-320.jpg)


![EXAMPLES
• The name of the following
coordination compounds are:
• (a) [Co(NH3)5Cl]Cl2 - pentaamminechlorocobalt(III)
chloride
• (b) [Fe(acac)3] - tris(acetylacetato)iron(III)
• (c) [Pt(en)2Cl2]Cl2 -
dichlorobis(ethylenediamine)platinum(IV) chloride,
• (d) [Ru(bpy)2Cl2] -](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-62-320.jpg)
![NOMENCLATURE: IUPAC RULES
• [Co(OX)3]3-
• Trioxalatocobaltate(III) ion
• K4[Fe(CN)6]
• Potassium hexacyanoferrate(II)](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-63-320.jpg)
![• Try this
• [Co(en)2(H2O)(CN)]Cl2
• Na2[MoOCl4]
• [Cr(H2O)4Cl2]Cl
• K4(Ni(CN)4]
NOMENCLATURE: IUPAC RULES](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-64-320.jpg)

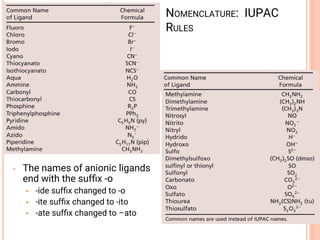
![• Define the following terms in coordination chemistry:
• (i) complex ion
• (ii) ligand
• coordination number
• Name the following complex ions
• (i) [Cr(H2O)6]3+ (ii) K2[TiCl6] (iii) [Pt(H2O)4(C2O4)]Br
2 (iv) [Co(NH3)6]Cl3 (v) [Co(H2NCH2CH2NH2)2Cl2]Cl
• State the oxidation number and the coordination number
of the metal in each of these complexes above](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-66-320.jpg)
![WRITING OF FORMULARS
• Tetraaminecopper(II) ion
Step 1: (NH3)4
Step2: Cu(NH3)4
Step 3: calculate the charge on the complex
i.e 2+0 =2
• [Cu(NH3)4]2+](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-67-320.jpg)

![ANSWERS
• [PtCl(NO2)2(NH3)3]+
• Na3[Co(NO2)6]
• [Fe(H2O)6]3+](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-69-320.jpg)

![CLASSWORK II
• 2. Write the IUPAC names of the following
coordination compounds and predict
• possible shape(s), hybridization(s) and bond
angle(s) of each::
• (i) [Co(NH3)6]Cl3
• (ii) [Co(NH3)5Cl]Cl2
• (iii) K3[Fe(CN)6]
• (iv) K3[Fe(C2O4)3]
• (v) K2[PdCl4]
• (vi) [Pt(NH3)2Cl(NH2CH3)]Cl](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-71-320.jpg)





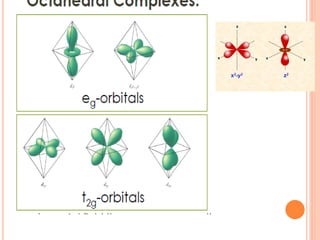
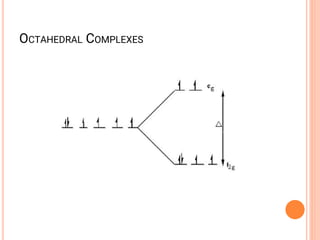
![CFT
• The crystal field model allows us to account for the
differences in the magnetic properties of
complexes. For example, [Co(NH3)6]3+ and [CoF6]3-
• [Co(NH3)6]3+ is known to be diamagnetic while
[CoF6]3- is know to be paramgnetic because it has
four unpaired electrons](https://image.slidesharecdn.com/chm003lecturenotetransitionmetal-240317192502-5407a596/85/CHM-003-Lecture-note-Transition-Metal-pdf-82-320.jpg)