This document provides information about the characteristics of d-block elements, also known as transition elements. It discusses their electronic configuration, variable valence, magnetic properties, catalytic properties, and ability to form complexes. It describes the first, second, and third transition series and provides examples of common oxidation states for elements in each series. The document also discusses the importance of d-block elements in applications such as metals, magnets, batteries, paints and more. It provides tables of typical oxidation states for different transition element groups.











![• Exercise:
• 1. The correct ground state electronic configuration of chromium Atom is
• A) [Ar]3d54s1 B) [Ar]3d54s1 C) [Ar]3d54s1 D) [Ar]3d54s1](https://image.slidesharecdn.com/dblock-200822141957/85/Dblock-12-320.jpg)



![• The electrons that lost depends on Internal electronic repulsions and variation in effective nuclear
charge.The energy level of 4s< 3d.so in the first transition series 4s orbital is filled first and then 3d
orbitals are filled.Hence,in the first transition series, ions should be formed by the loss of 3d electrons
rather than 4s electrons .Actually this is not true. In fact atoms of first transition series lose 4s electrons
before they lose electron from 3d orbitals. The reason for this behaviour is that after the electrons have
entered 3d orbitals the energy of 3d orbitals becomes less than that of 4s orbital. For example the
electronic configuration of titanium(Z=22) will be as Ti:[Ar]3d24s2
• Therefore electronic configuration of Ti 2+ will be as Ti2+ : [Ar] 3d2
• Similarly for electronic configuration of Ti 3+ will be as Ti3+ : [Ar] 3d1
• The transition elements have their valence electrons in two different sets of orbitals that is (n-1)d and ns.
As there is very little difference in the energies of these orbitals, both energy levels can be used for bond
formation.
• In simple compounds the two electrons from ns orbital of a transition element are used to give an
oxidation state of +2 and the (n-1)d electrons remain unaffected.
• The higher oxidation states like +3, +4, +5, +6 and +7 correspond to the use of all 4s and 3d electrons in
the transition series of elements.
• In excited state, the (n-1)d electrons become bonding and thus give variable oxidation states to the atoms
of transition elements.
• Thus, transition elements show variable valencies due to involvement of penultimate d shell electrons.](https://image.slidesharecdn.com/dblock-200822141957/85/Dblock-16-320.jpg)







![The +II and +III states are important for all the first row transition elements.
Simple ions M2+ and M3+ are common with the first row but are less important for second and 3rd row elements
,which have few ionic compounds. Similarly the first row form a large number of extremely stable complexes such
as [CrCl6]3-and [CO(NH)3]6 .No equalent complexes of Mo or W or Rh or Ir are known.
The higher oxidation states of the second and 3rd row elements are more important and much more stable than
those of the first row elements .
Thus the chromate ion[CrO4]2- is a strong oxidising agent but molybdate [MoO4]2- and tungstate [WO4]2- are stable.
similarly the Permanganate ion [MnO4]- Is a strong oxidizing agent but pertechnetate [TcO4]- and perrhenate
[ReO4]- ions are stable.
some compounds exist in high oxidation states which have no counterparts in the first row. for example wCl 6, ReF
7, RuF4,OsO4 and PtF 6 .
+2,+3 form ionic compounds
Higher o.s form covalent compounds
The o.s of metal in solvents depend on solvent’s nature.
Ex:
Cu+1 in H2O---unstable
Cu+2 in H20---Stable.
With F&O—high o.s(the stability of particular oxidation state depends on the nature of the combining elwement)
The relative stabilities of o.s is known from std.electrode potentials.](https://image.slidesharecdn.com/dblock-200822141957/85/Dblock-24-320.jpg)

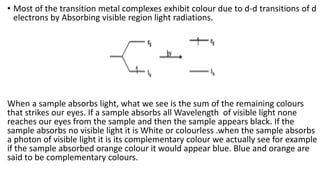






![• Complex compounds are compounds wherein a number of neutral molecules or
anions are bound to a metal. Metals which are a part of the d block elements
form many complex compounds owing to their small ionic size, high charge,
and relative availability of d orbitals for the formation of bonds.
• Transition metal and their ions with their larger nuclear charge and smaller size
can attract electrons and receive lone pair of electrons from anions and neutral
molecules into their empty d-orbitals forming coordinate bonds.
• Transition elements thus form complex molecules with CO, NO, NH3, H2O, F–,
Cl–, CN–. Examples of transition metal complexes are
[Co(NH3) 6] 3+ [Cu(NH3)4] 2+, Y(H2O) 6]2+, [Fe(CN)6]4−, [FeF6] 3−, [Ni(CO)4]
The coordination number 6 is widespread in the transition elements giving an octahedral
structure .The coordination number four is much less common giving tetrahedral and square
planar complexes .
Coordination no. of 7 and 8 are uncommon for the first row but are much more common in
the other members of the second and 3rd rows.](https://image.slidesharecdn.com/dblock-200822141957/85/Dblock-33-320.jpg)























![• Complex formation:
• The tetrahalides of these elements act as Lewis acids with a wide variety of
donors, forming and large number of stable octahedral complexes.
• TiF4 [TiF6]2-
• TiCl4 [TiCl6]2-
• On going down the group the size of M4+ ion increases and the tendency of the
elements to form complexes decreases .
• Hence Ti 4+ form complexes more easily than Zr4+ and Hf 4 +
.
• Colour and magnetism:
• M4+ ions with d0 configuration are colourless and diamagnetic.
• M3+ ions with d1 configuration are coloured and paramagnetic.
• Ex:[Ti(H2O)6]3+ is violet due to unpaired electron.
Conc.HF
Conc.HCl](https://image.slidesharecdn.com/dblock-200822141957/85/Dblock-57-320.jpg)






![• Complexes:
• Cu(II) Forms stable complexes like [Cu(CN)4]2-(square planar) and [CuCl4]2-
(tetrahedral ).
• Ag(I) forms Stable compounds like AgCl.It forms ammine complexes like
[Ag(NH3)2]+.](https://image.slidesharecdn.com/dblock-200822141957/85/Dblock-64-320.jpg)