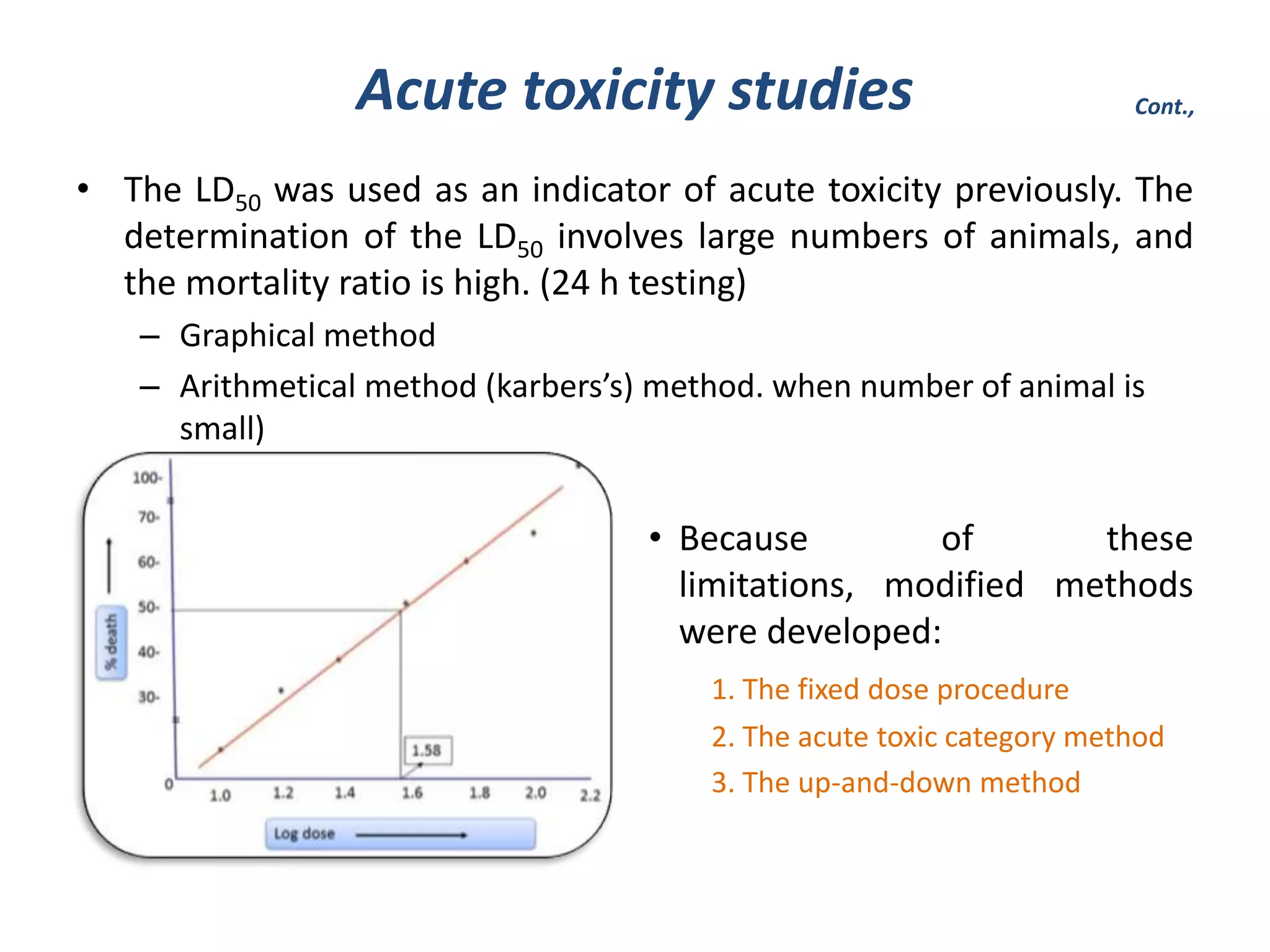
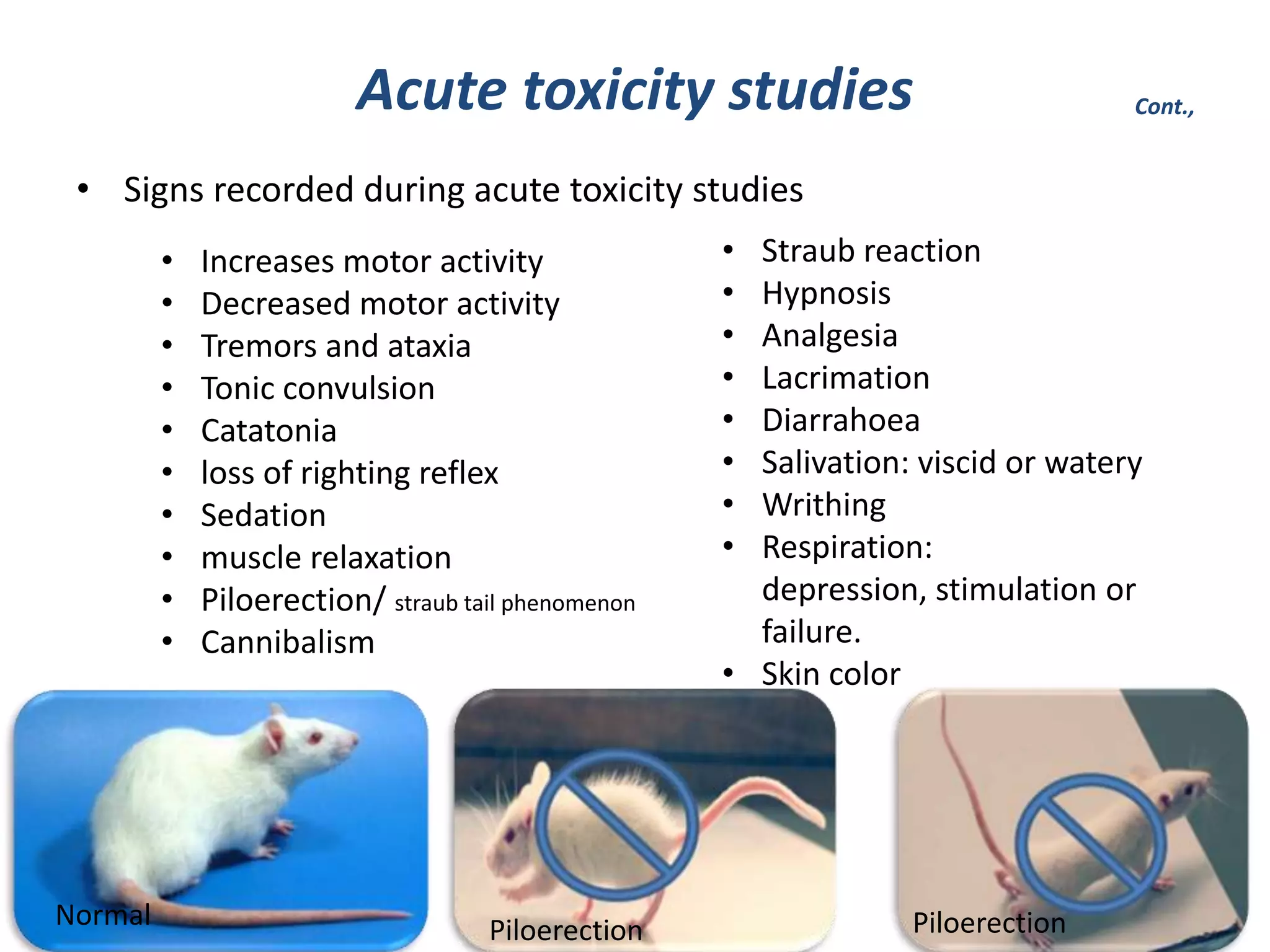
Toxicity studies are important for developing new drugs and extending the use of existing drugs. They help determine the potential pharmacological effects and toxicity of new molecules in animals according to FDA guidelines. Toxicity tests examine adverse events like cancer, organ toxicity, and skin/eye irritation. They also help calculate the no observed adverse effect level dose for clinical trials. Acute, subchronic, and chronic toxicity studies in rodents and nonrodents are commonly used to identify target organs of toxicity and determine safe dosage levels over different time periods. Proper ethics approval is required before conducting any animal studies.