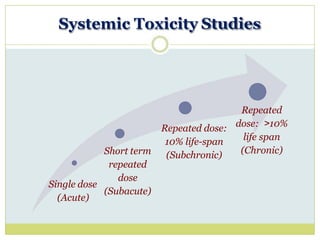
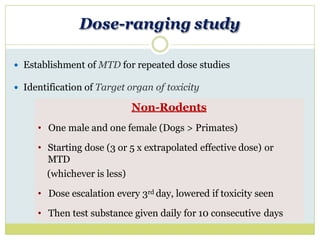
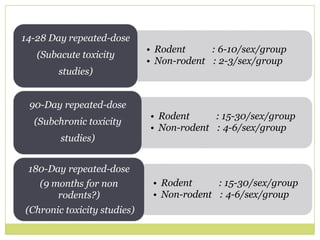
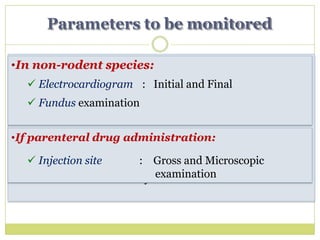
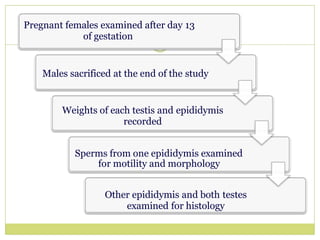
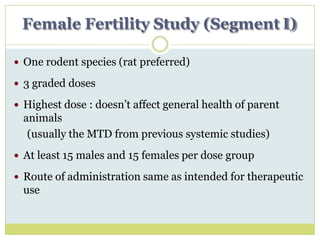
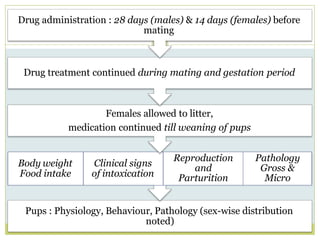
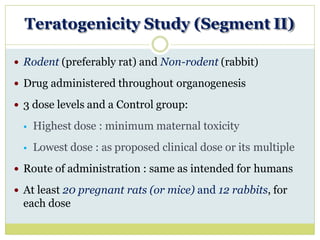
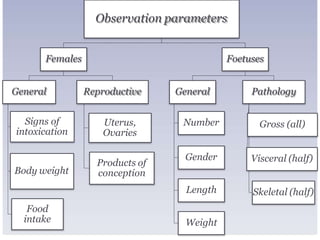
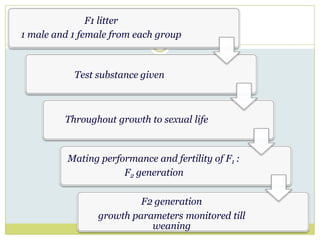
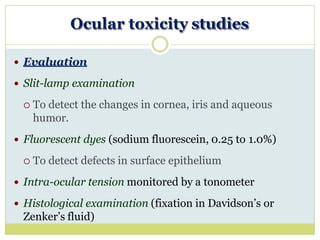
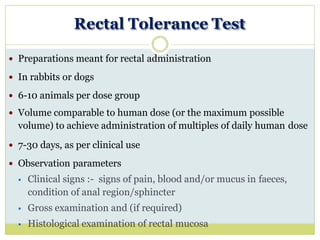
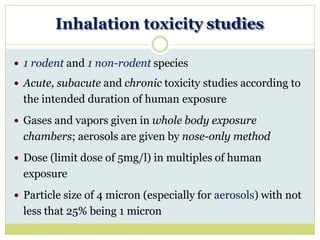
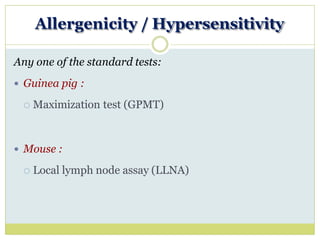
The document outlines the course ZOL-610 in Environmental Toxicology, detailing its objectives, content, and the methodology for toxicity studies, including acute, subacute, and chronic toxicity assessments for various test substances. It emphasizes the importance of understanding the effects of environmental toxicants and the need for specific animal studies to evaluate toxicity and safety in potential drug development. The course also covers the evaluation of reproductive and local toxicity, as well as the procedures and parameters to be monitored throughout the testing process.











![Single-dose (Acute) Toxicity Studies
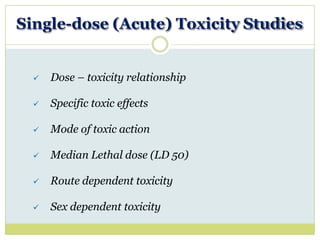
At least 5 animals / sex / group
At least 4 graded doses (4 dose-groups)
Observation for toxic effects (if any) : 14 days
Obsevation for mortality :
7 days [parenteral administration]
14 days [oral administration]](https://image.slidesharecdn.com/toxicitystudies-210202120124/85/Toxicity-studies-19-320.jpg)





![Repeated-dose Toxicity Studies
Wherever applicable, include a Control group
3 other groups are formed, as:
Highest dose
Lowest dose
Intermediate dose
Observable toxicity [MTD]
No observable toxicity
[NOAEL]
intended therapeutic dose or
multiple of it
Some symptoms ; not gross
toxicity or death placed
logarithmically betn doses](https://image.slidesharecdn.com/toxicitystudies-210202120124/85/Toxicity-studies-26-320.jpg)



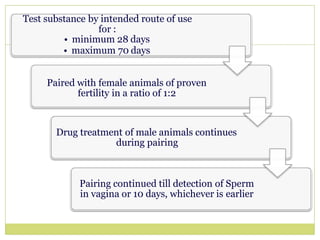









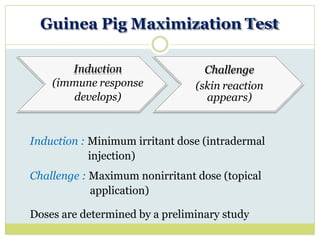

![Induction (Day 0)
3 pairs of intradermal injections on either shoulders :
• 0.1 ml Freund’s adjuvant alone
• 0.1 ml test material (lowest irritant dose)
• 0.1 ml test material in Freund’s adjuvant
Day 7 : Topical patch at prepared shoulders (lowest irritant dose)
Challenge (Day 21)
Topical patch at prepared flanks (highest non-irritant dose)
• Left side: Test agent
• Right side: Vehicle
Evaluation [Edema and Erythema] after 48 hr.](https://image.slidesharecdn.com/toxicitystudies-210202120124/85/Toxicity-studies-56-320.jpg)

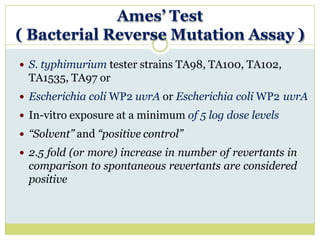
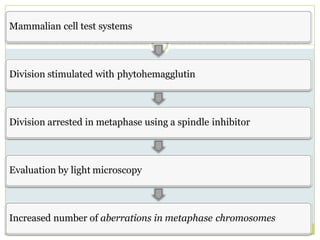
![In-vitro cytogenetic assay
Performed in CHO cells or on human lymphocyte in
culture
In-vitro exposure using a minimum of 3 log doses
“Solvent” and “positive control”
[Positive control : Cyclophosphamide / Mitomycin C
gives a reproducible and detectable increase in
clastogenic effect]
> 50% inhibition of cells is considered significant](https://image.slidesharecdn.com/toxicitystudies-210202120124/85/Toxicity-studies-61-320.jpg)
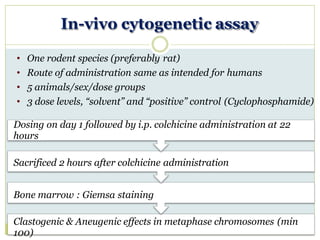
![In-vivo micronucleus assay
• 1 rodent species (preferably mouse) needed
• Route of administration of test substance same as humans
• 5 animals / sex / dose groups
• At least 3 dose levels, plus “solvent” and “positive” control
• Positive control : mitomycin C or cyclophosphamide
Dosing : day 1 and 2 of study
Sacrifice of animals 6 hours after the last injection
Bone marrow : Smeared with May Gruenwald / Giemsa stain
↑micronuclei in polychromatic erythrocytes [M-PCE] (min 1000)](https://image.slidesharecdn.com/toxicitystudies-210202120124/85/Toxicity-studies-66-320.jpg)