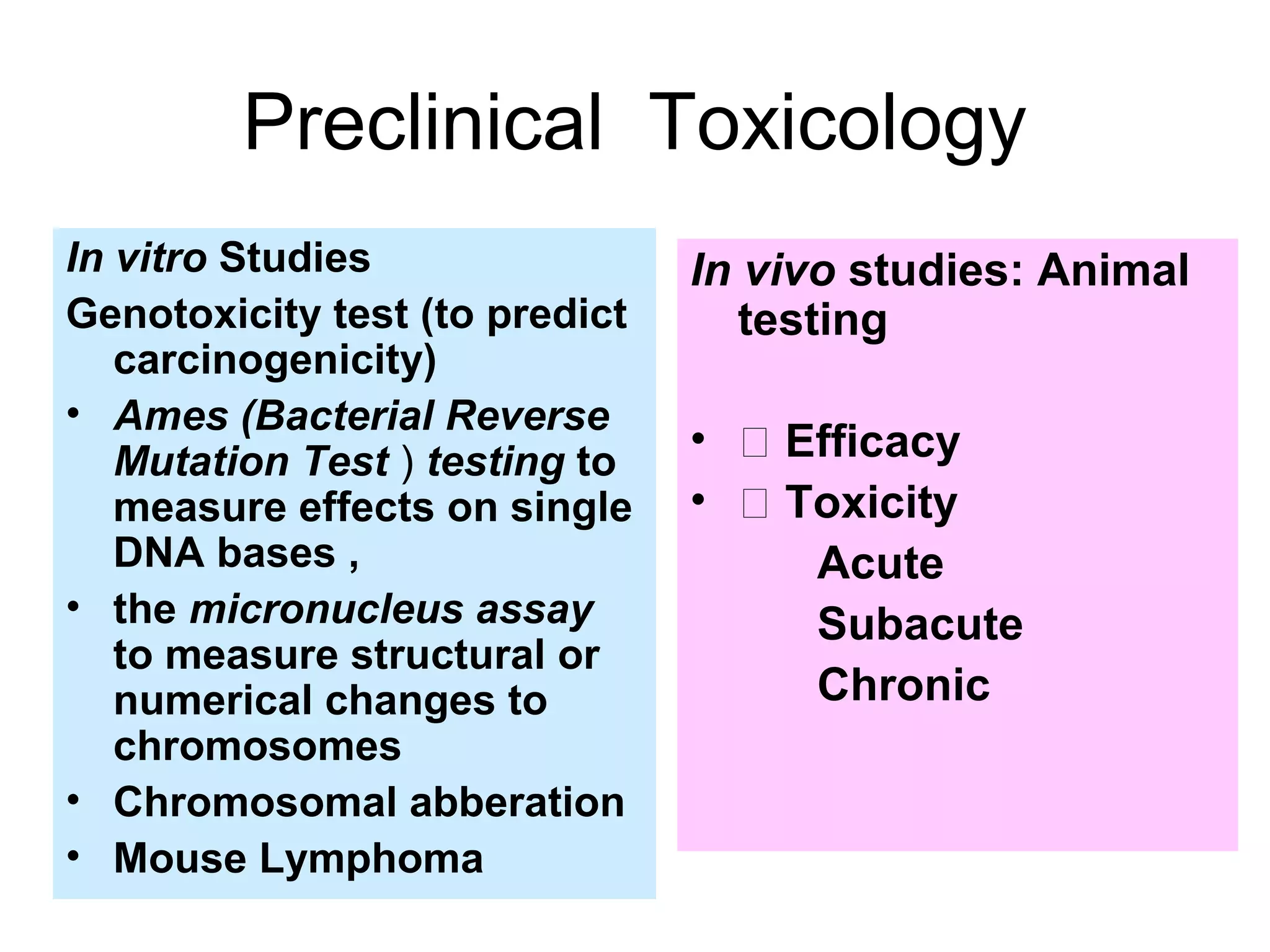
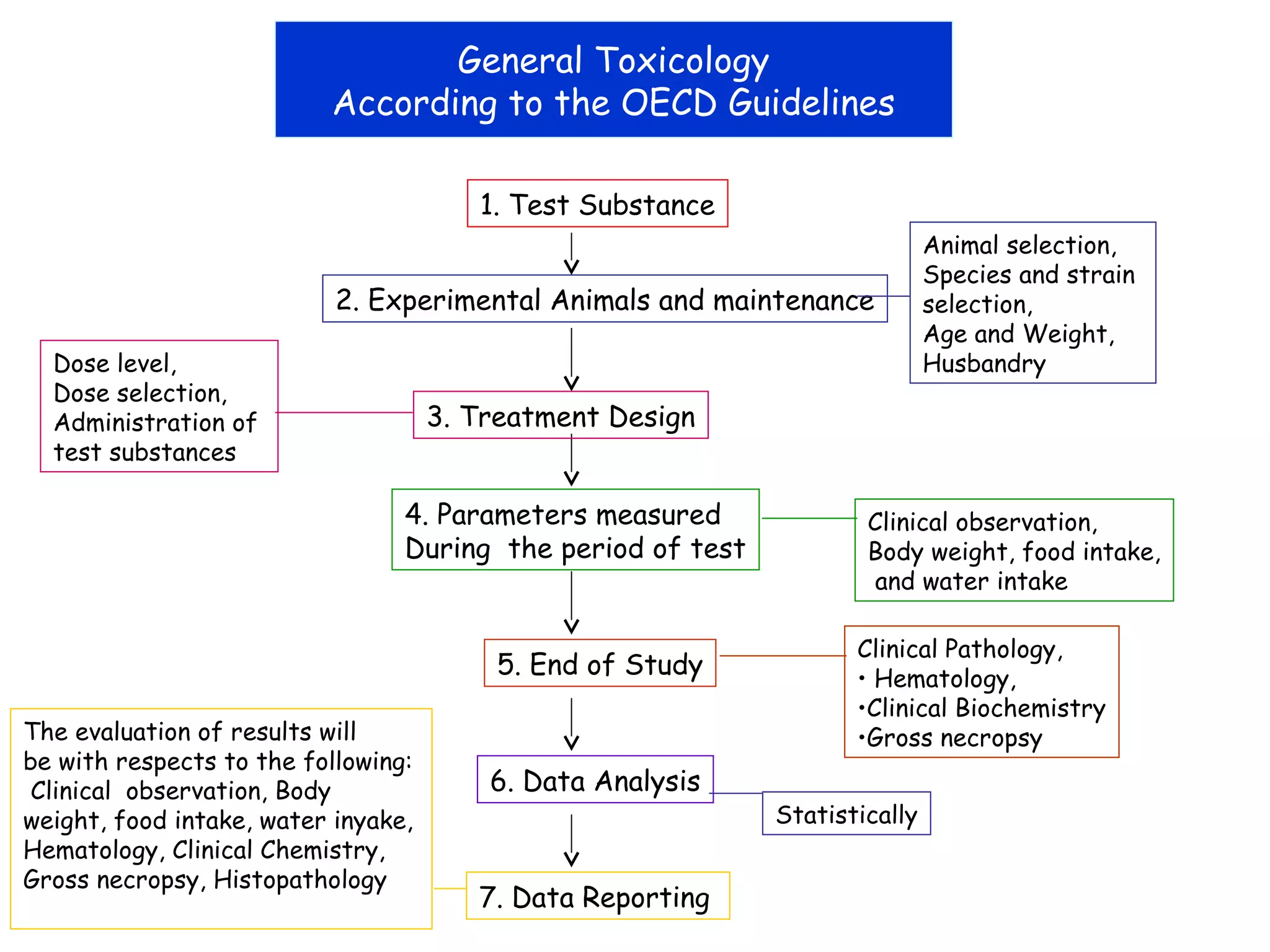
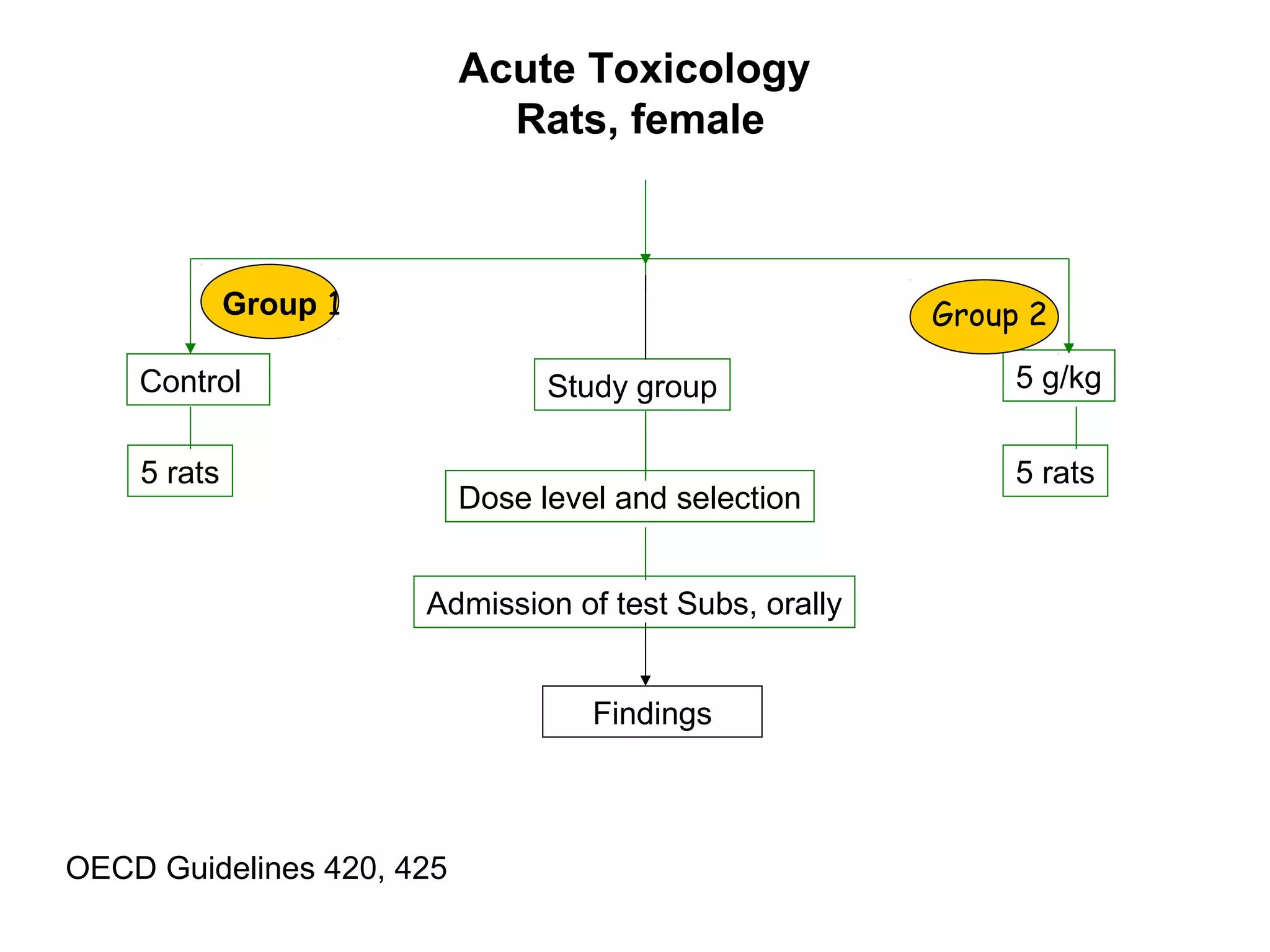
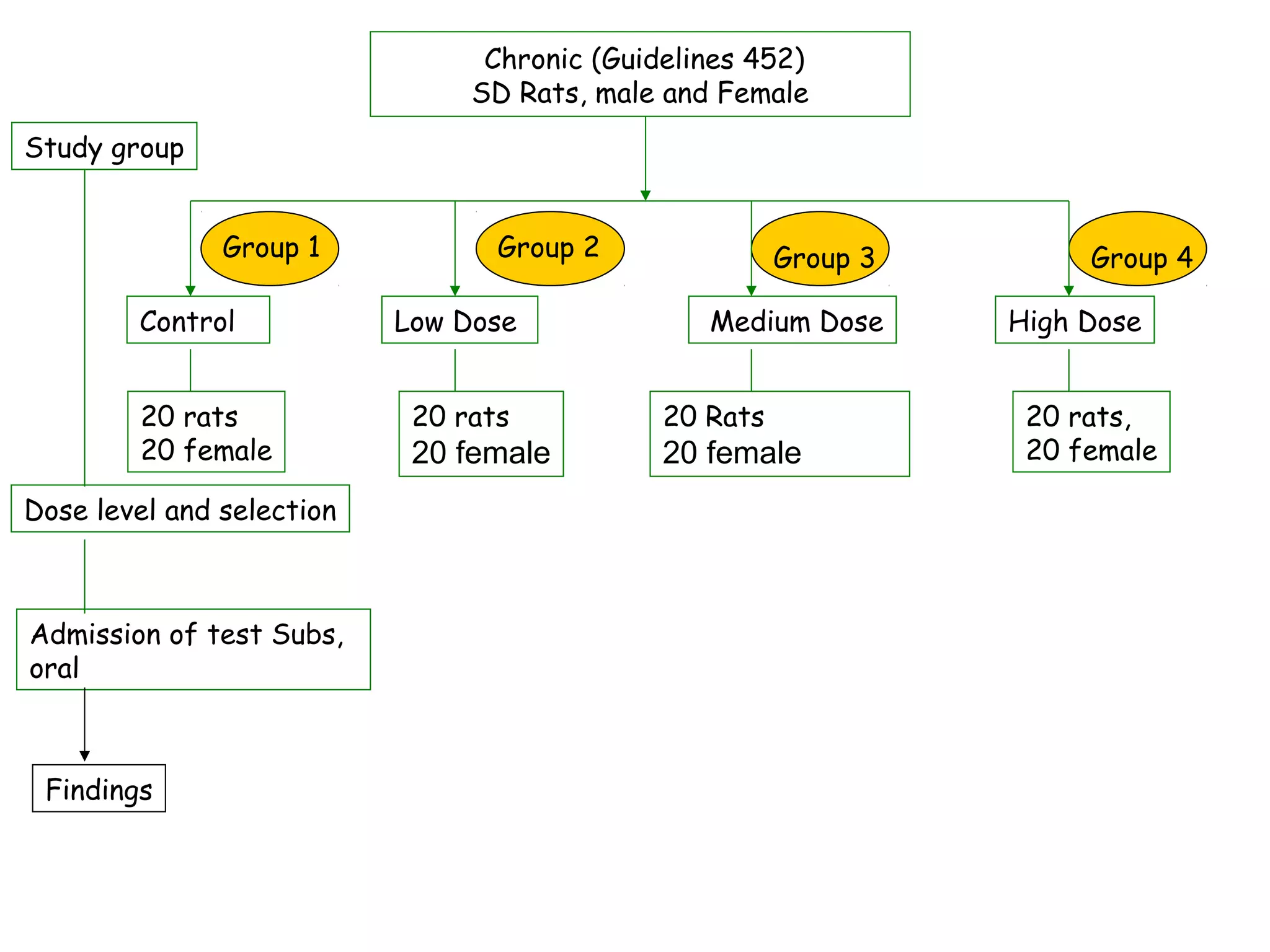
General toxicology testing refers to a series of toxicity tests required by international regulators to prove safety in experimental animals prior to human testing. It includes acute, sub-acute, and chronic toxicity tests conducted according to OECD guidelines in rodents and non-rodents. Preclinical studies include phytochemistry, formulation development, pharmacology/pharmacokinetic profiling, safety toxicology studies, and efficacy studies. Toxicology studies are guided by regulatory requirements like OECD/ICH guidelines and Good Laboratory Practices to ensure quality. Acute, sub-acute, and chronic toxicity tests provide information on toxicity effects from single or repeated substance exposure over different time periods and help determine safe doses for clinical trials.