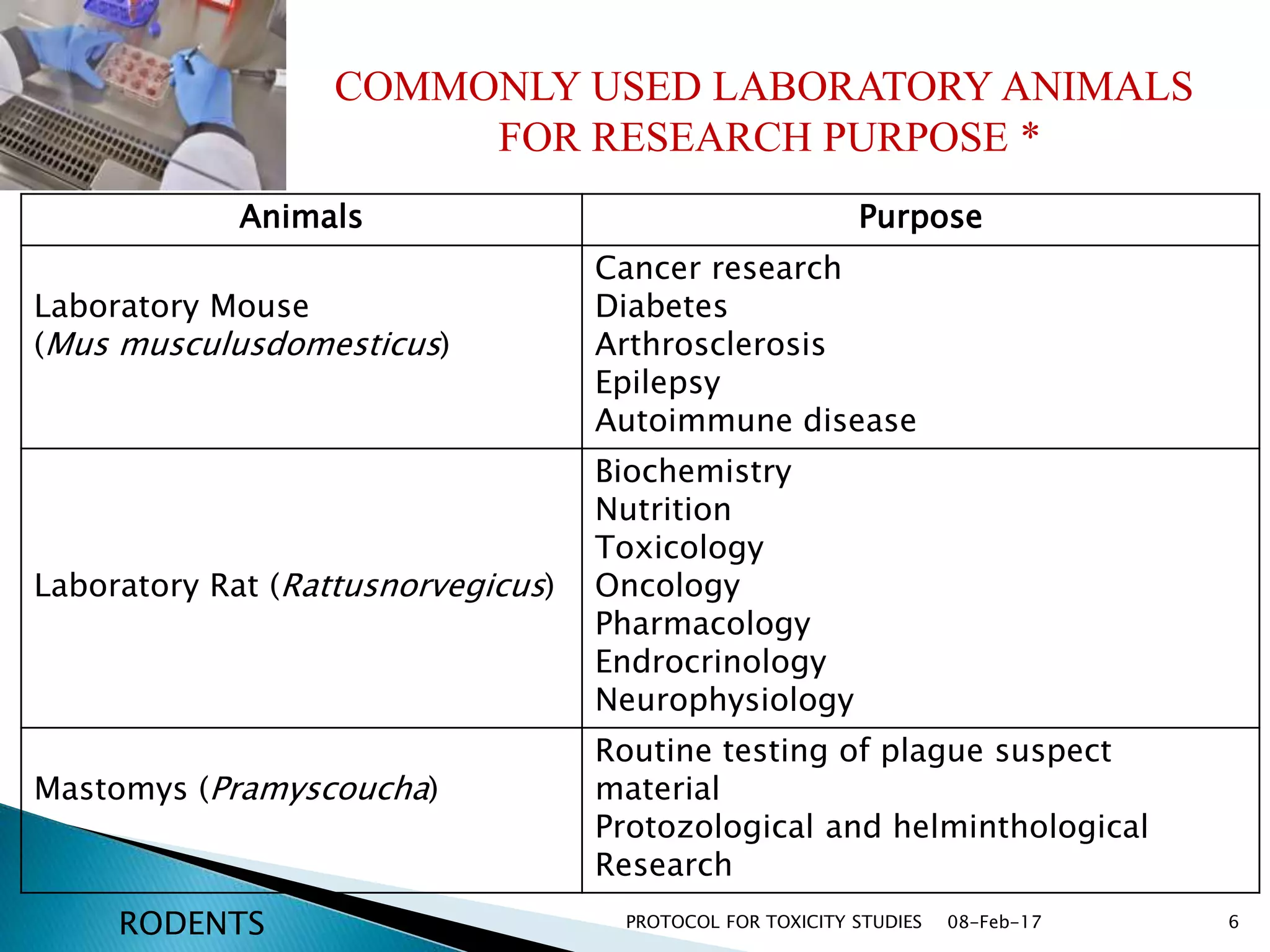
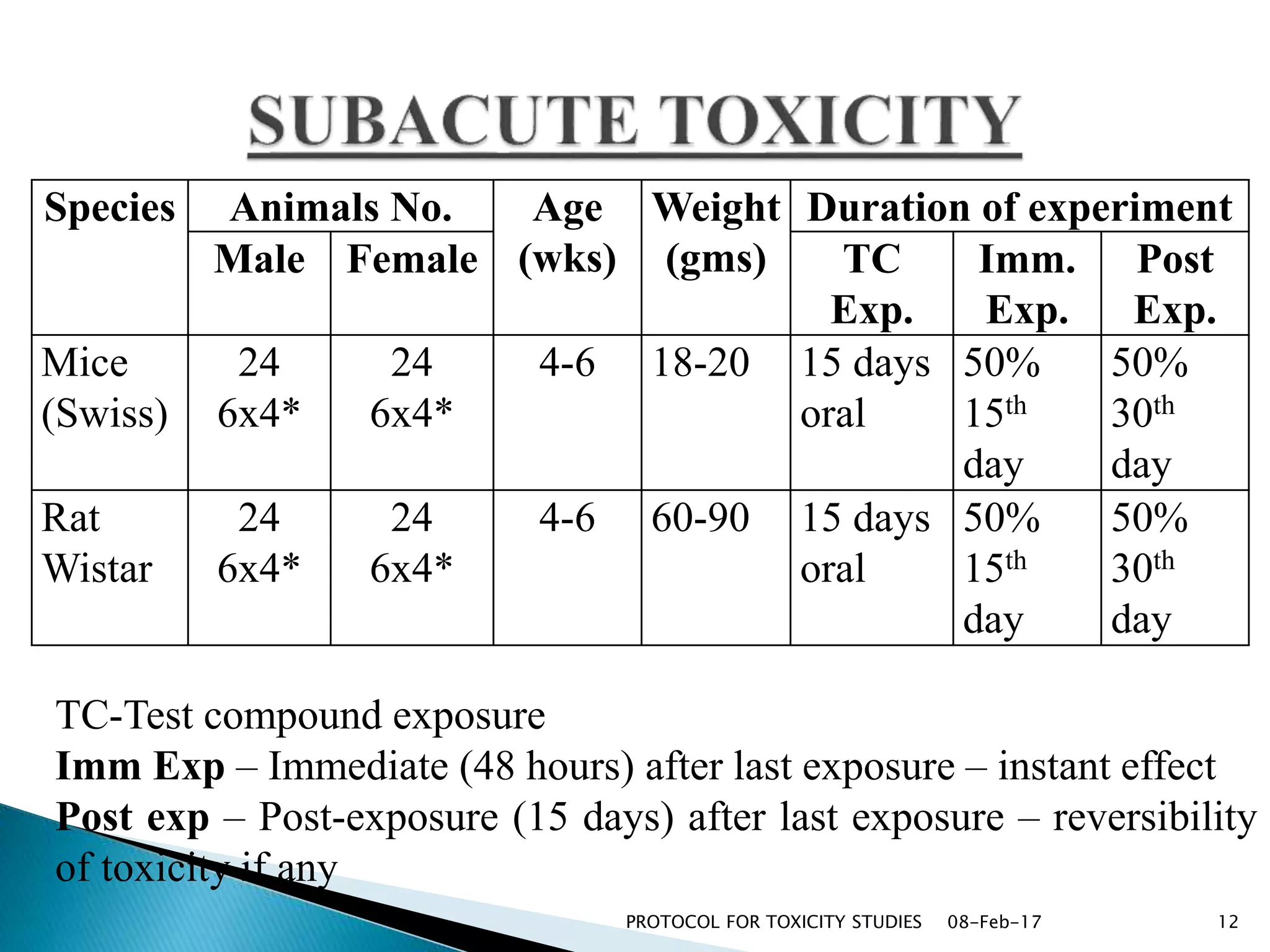
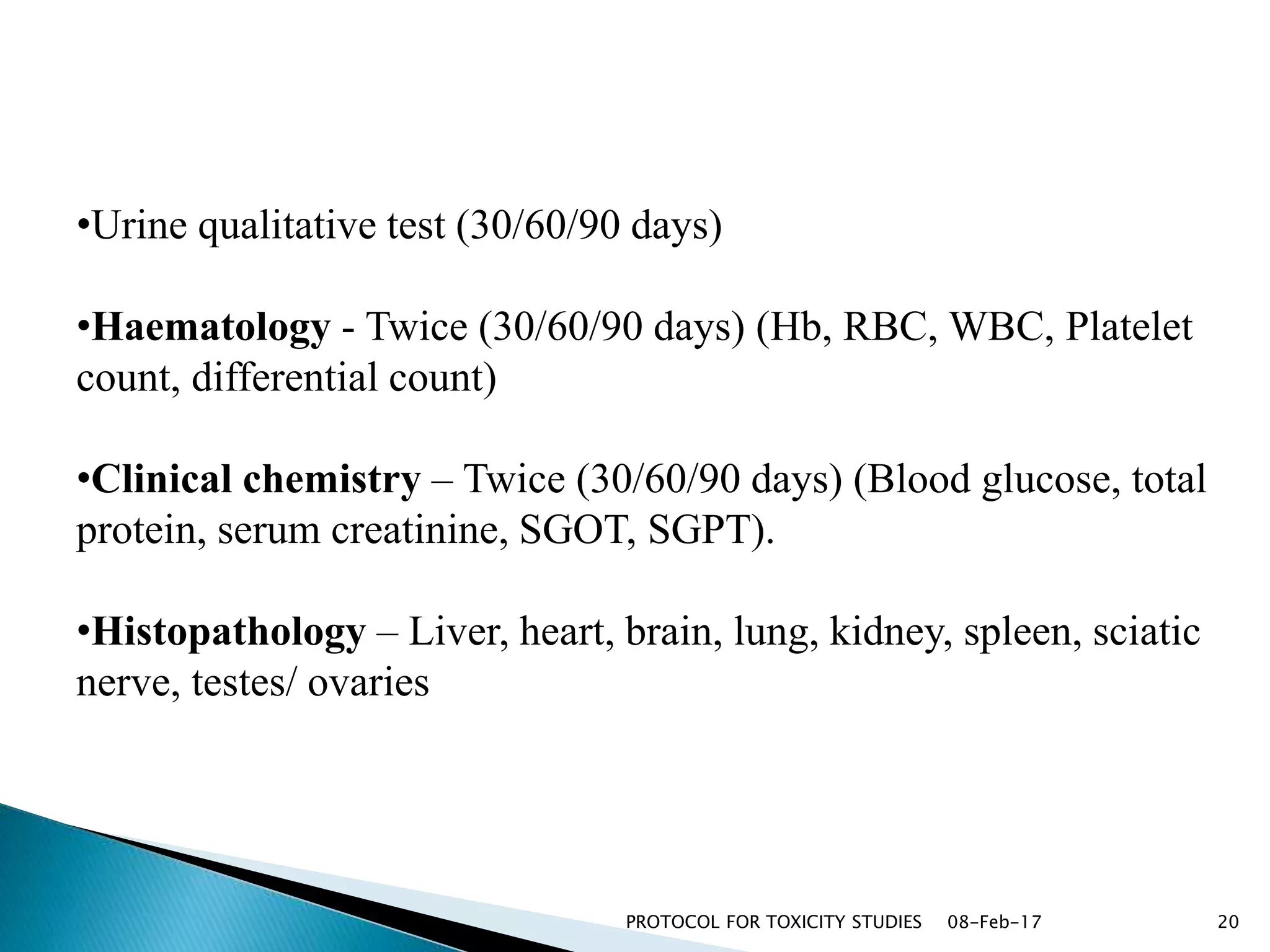
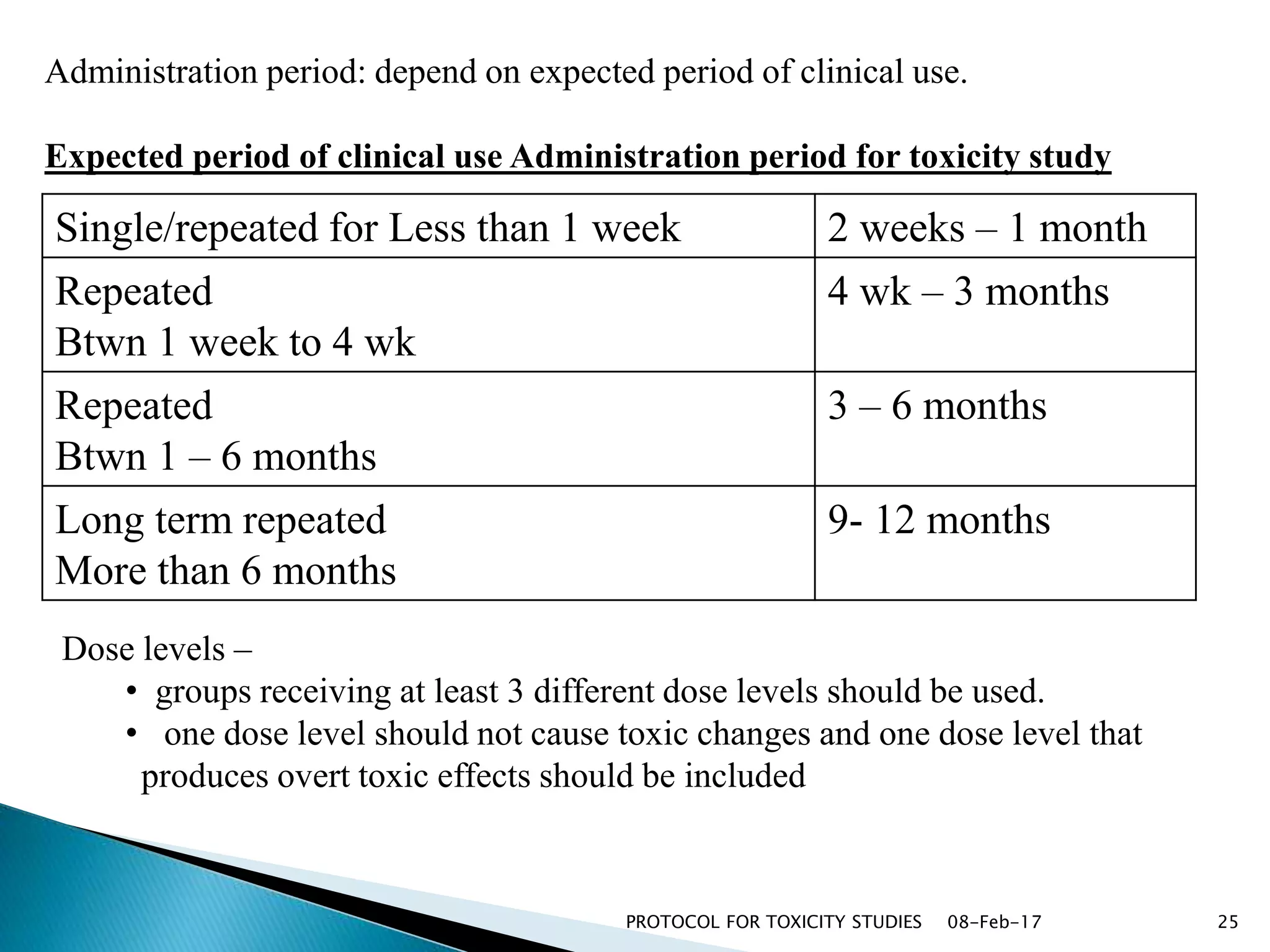
This document outlines protocols for conducting toxicity studies of herbal medicines. It discusses various types of toxicity studies including acute, sub-acute, chronic, and subchronic studies. It provides details on commonly used laboratory animals, testing parameters, dosages, and observations for each type of study. The goal is to safely assess herbal medicines and determine appropriate dosage levels for clinical use by evaluating toxicity signs and examining tissue samples.