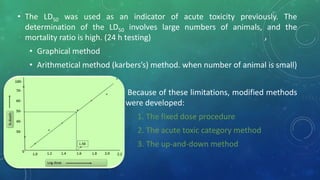
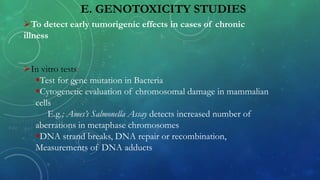
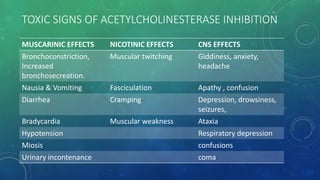
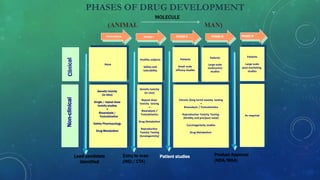
This document provides an overview of toxicity testing methods for acute, subacute, and chronic toxicity studies. It discusses the importance and history of toxicity testing, as well as standard methods and guidelines established by organizations like OECD and EPA. A variety of in vivo and in vitro toxicity tests are described, including acute, repeated dose, genotoxicity, carcinogenicity, and local toxicity studies. The document also addresses the large number of animals used annually for toxicity testing globally and the regulatory framework for animal testing in India.