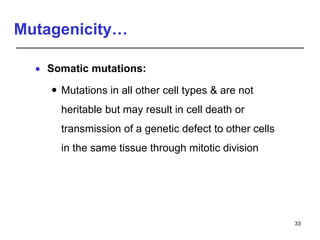
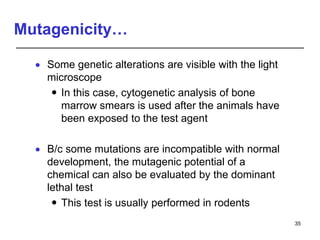
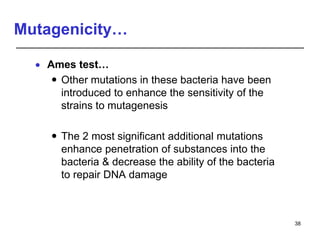
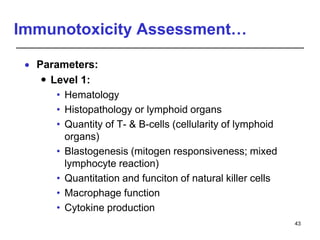
Studies on general toxicity include acute, sub-acute, sub-chronic, and chronic toxicity studies to determine the effects of repeated exposure to a chemical over different time periods. Developmental and reproductive toxicity studies evaluate effects on fertility, pregnancy, and offspring. Mutagenicity studies test whether chemicals cause genetic mutations. Carcinogenicity studies involve long-term exposure of rodents to assess cancer potential. Immunotoxicity assessment evaluates impacts on the immune system.