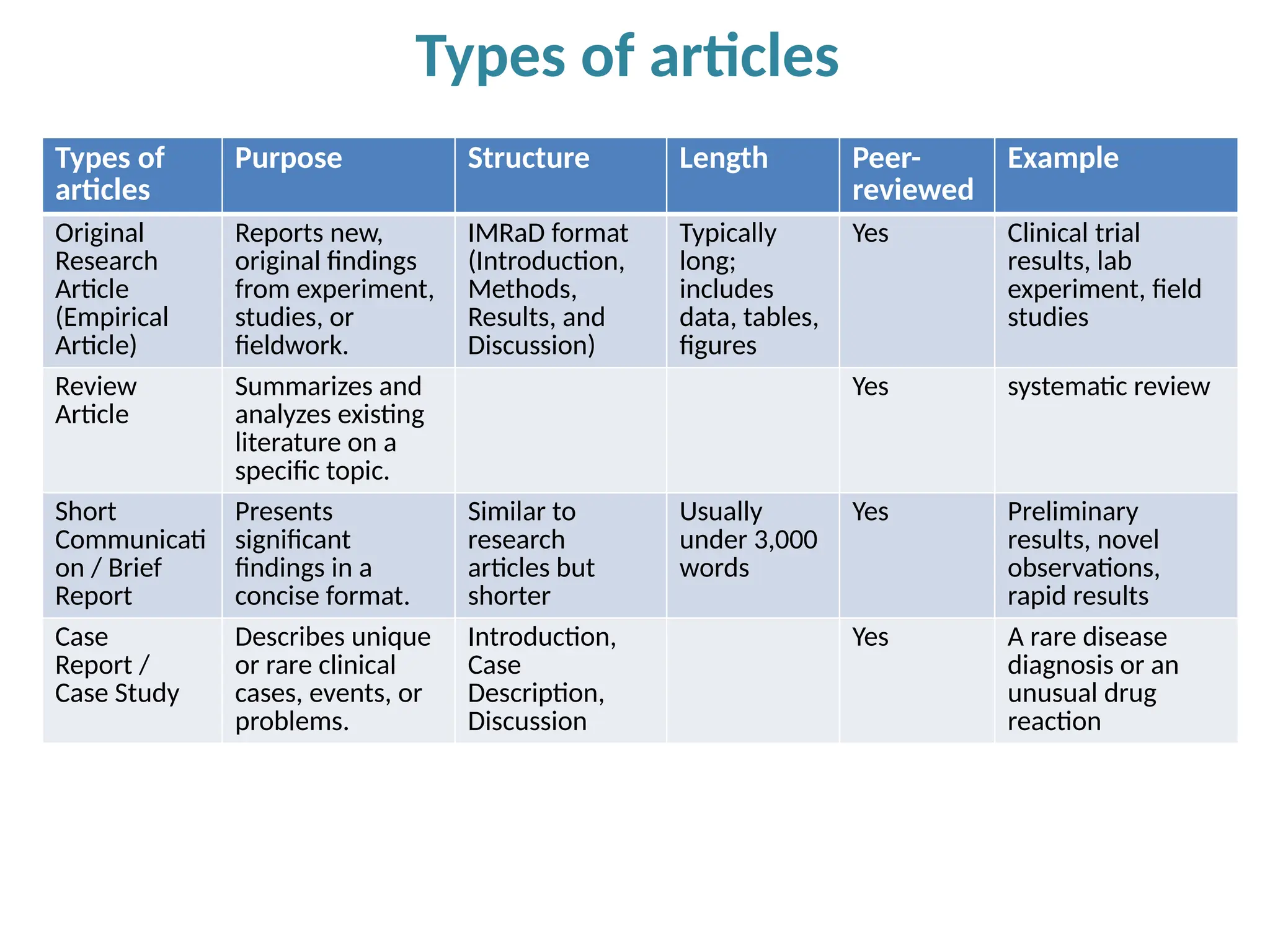
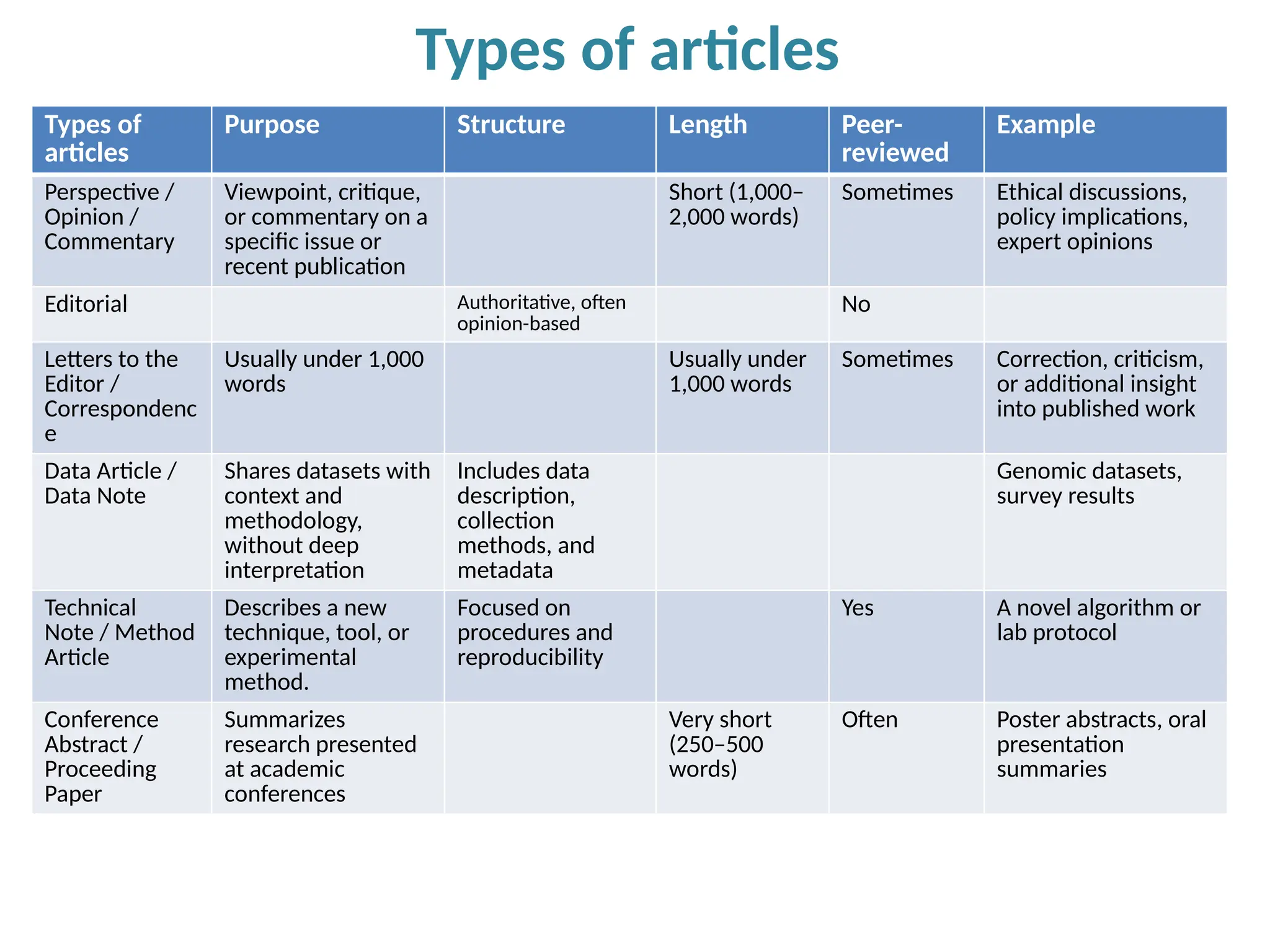
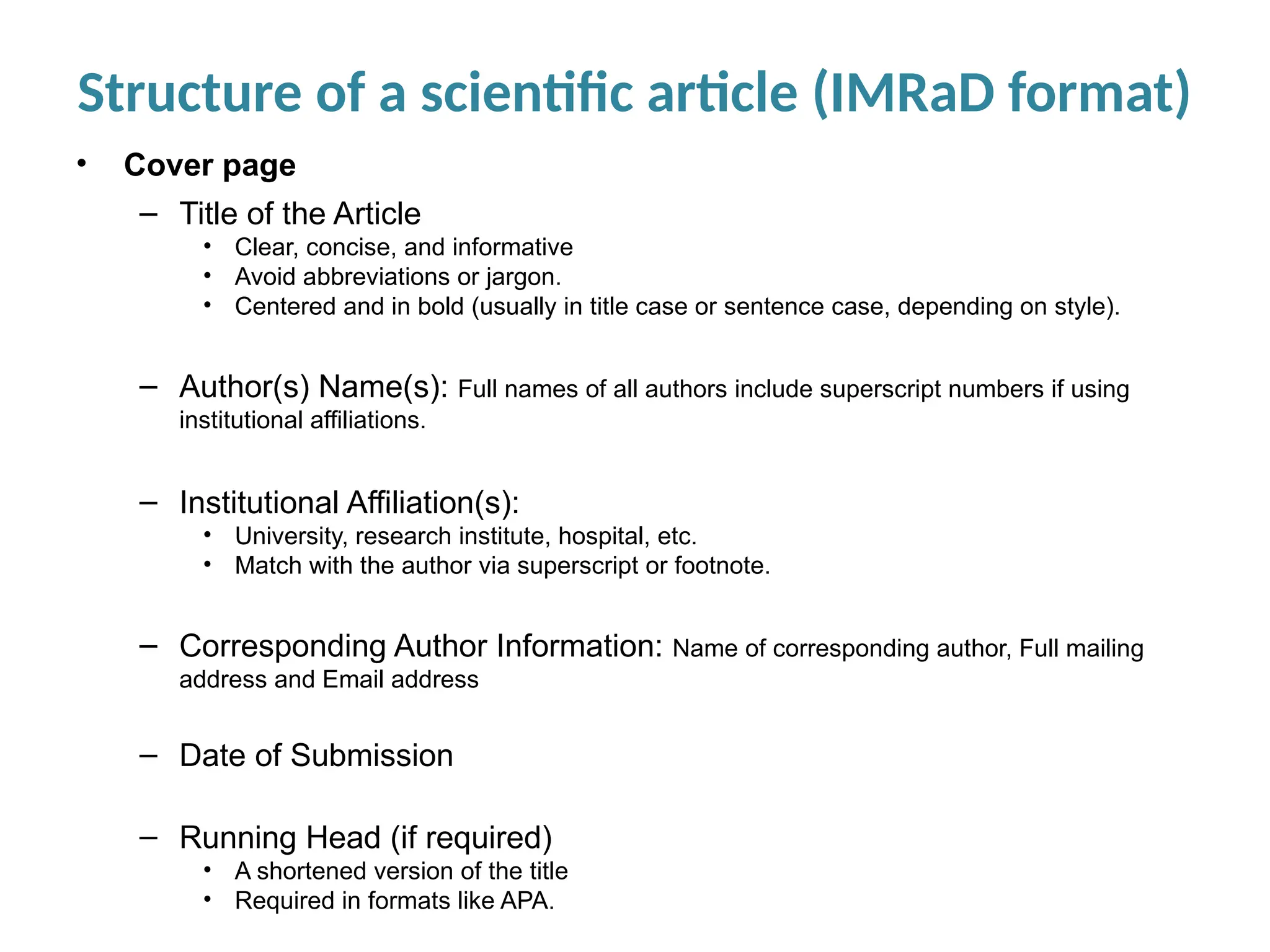
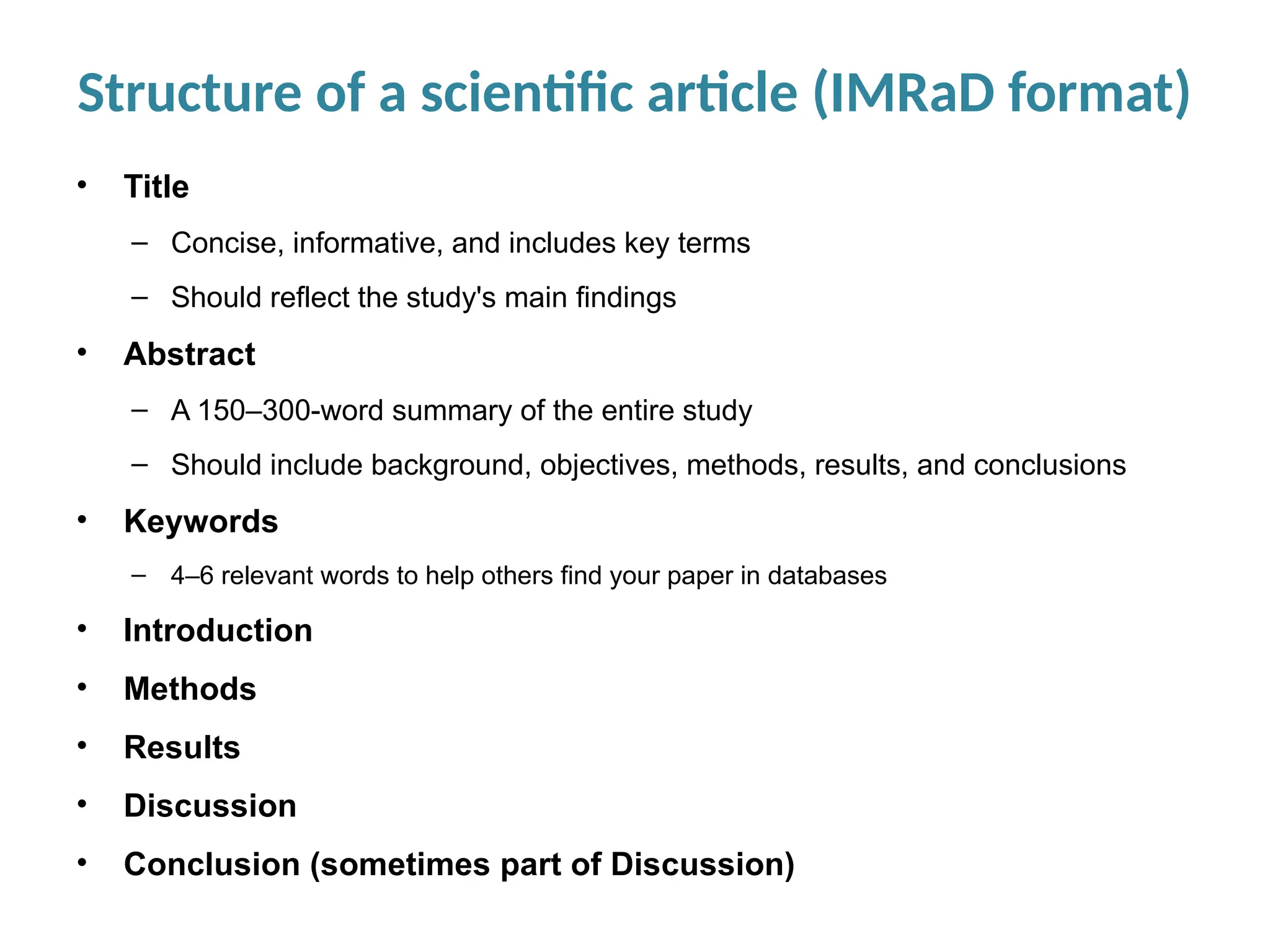
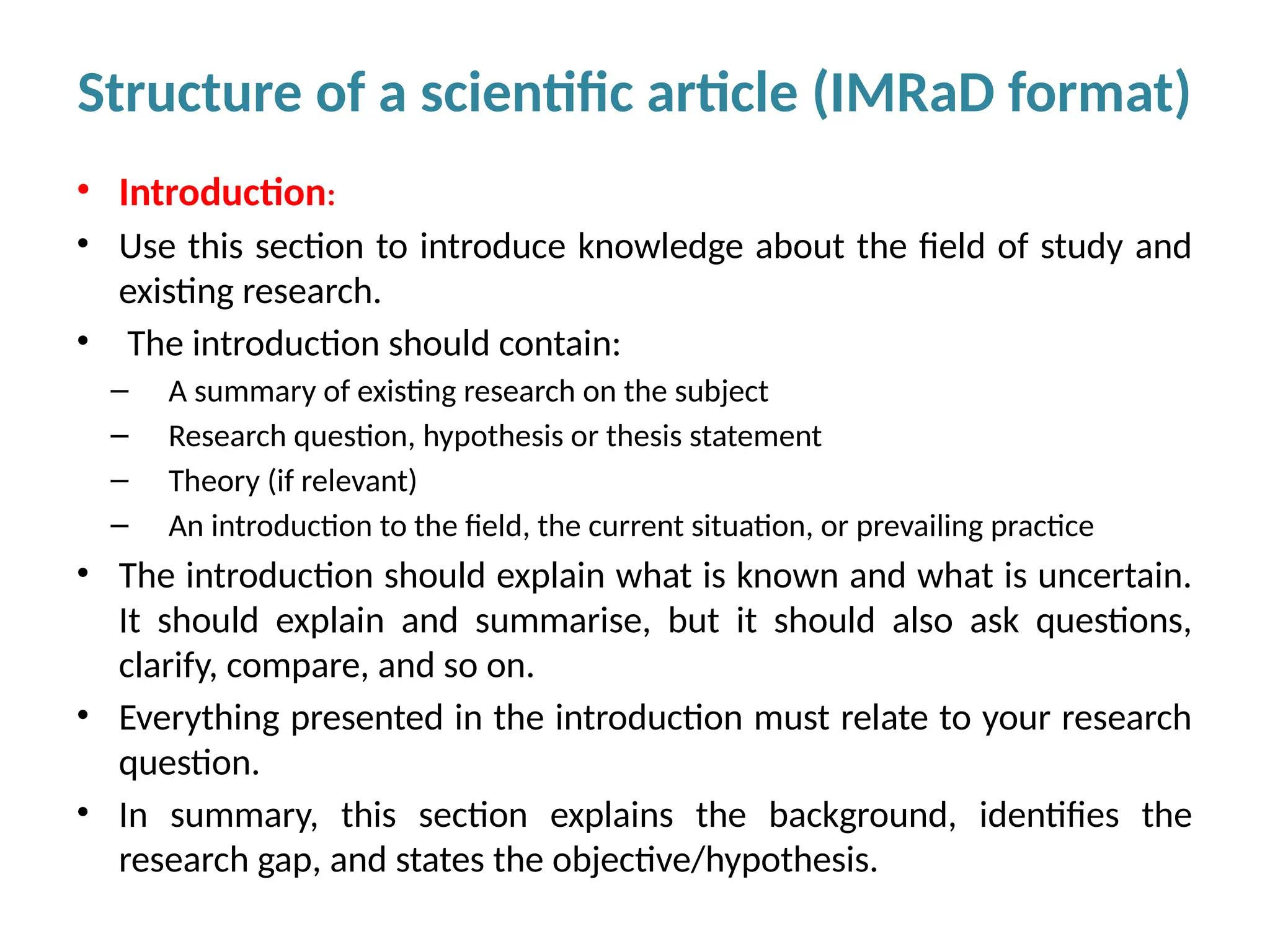
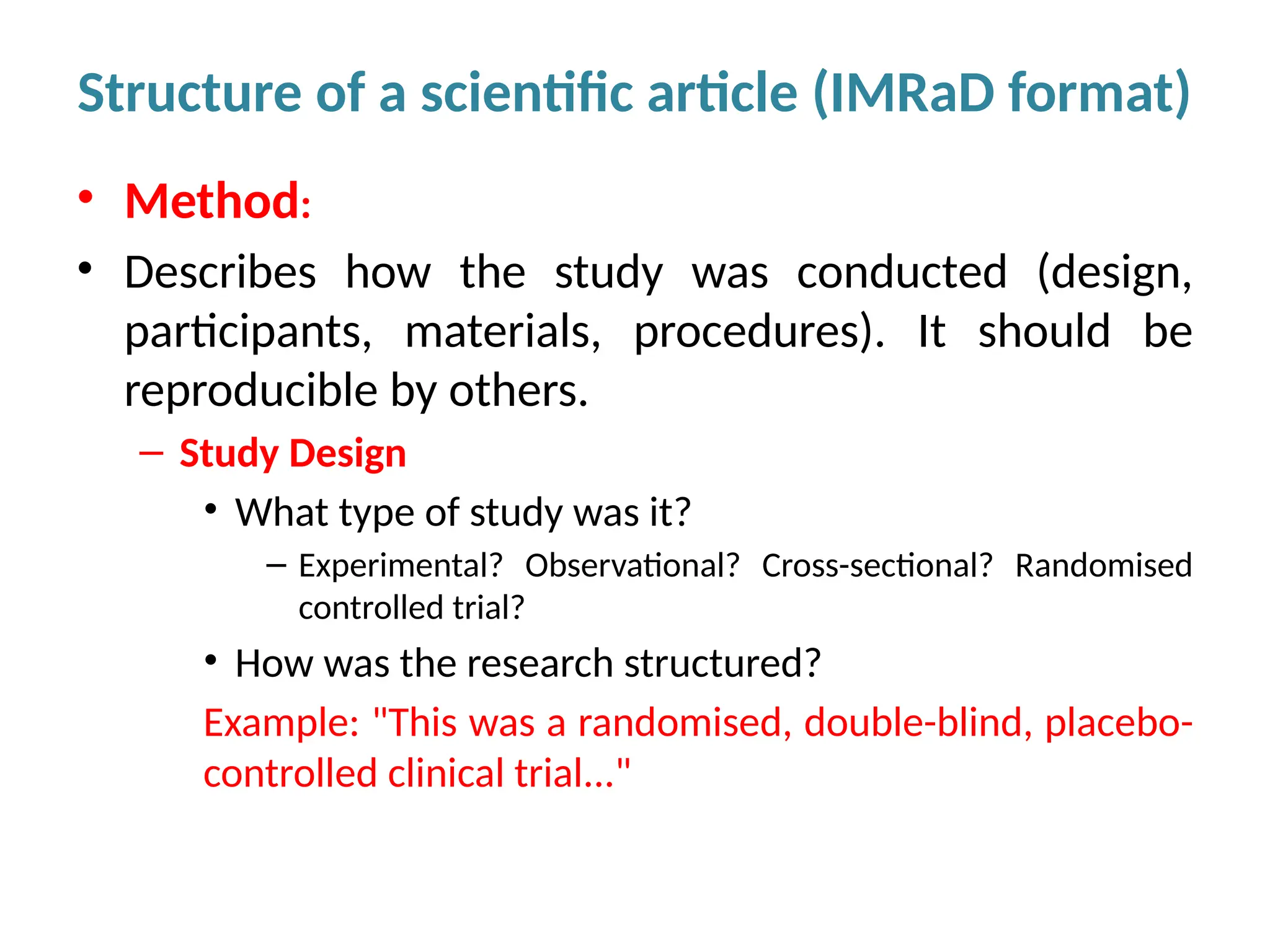
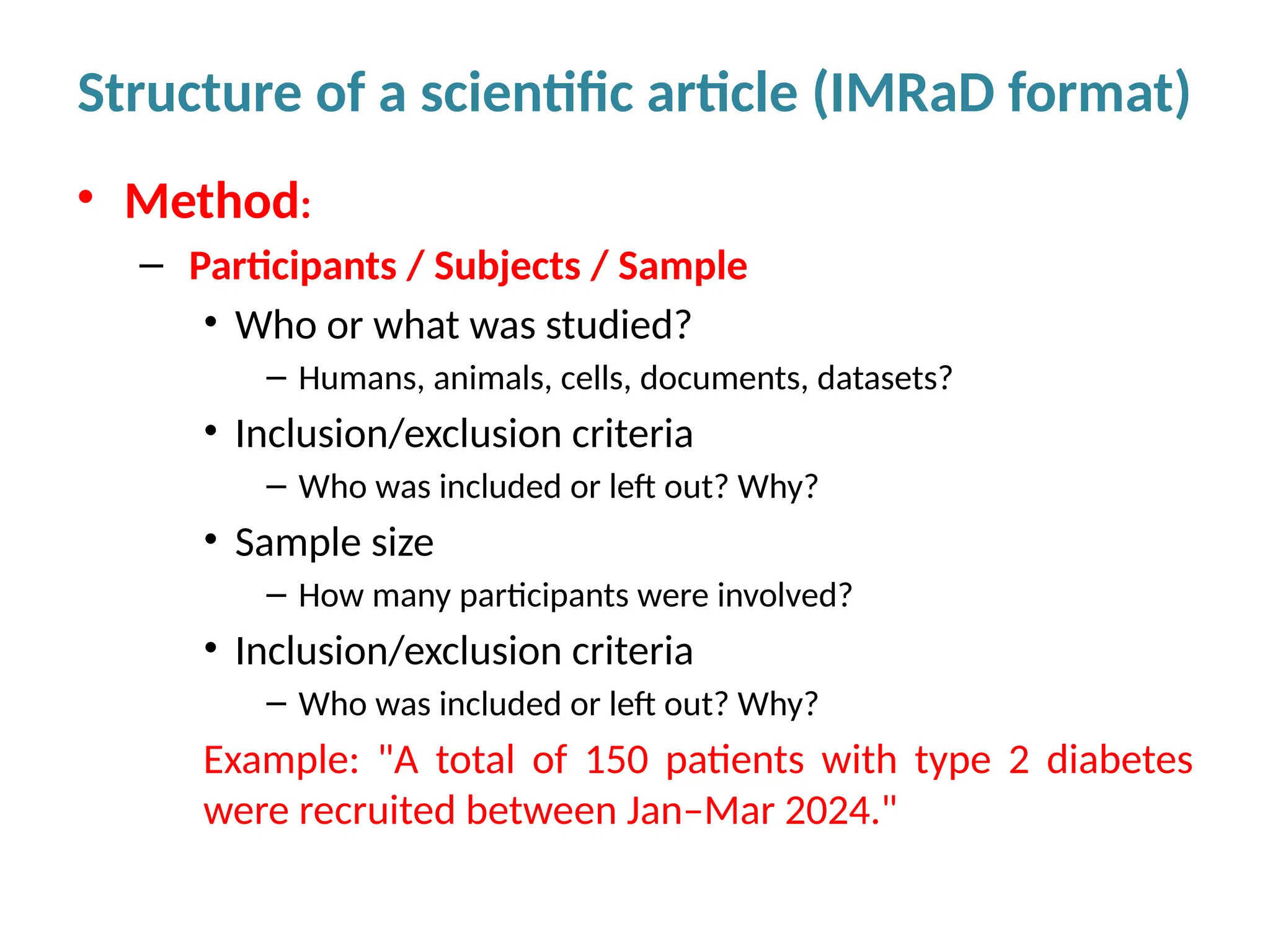
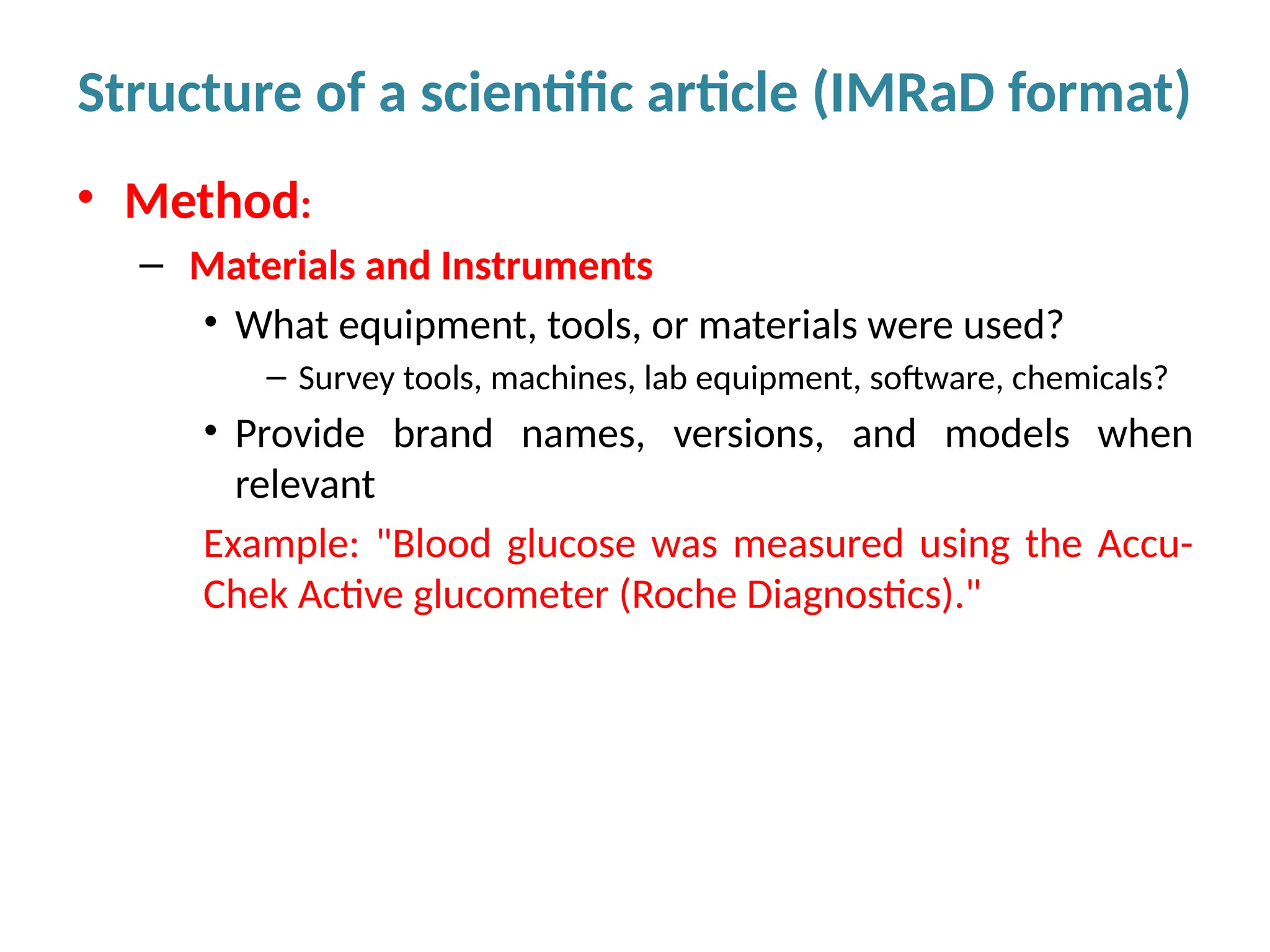
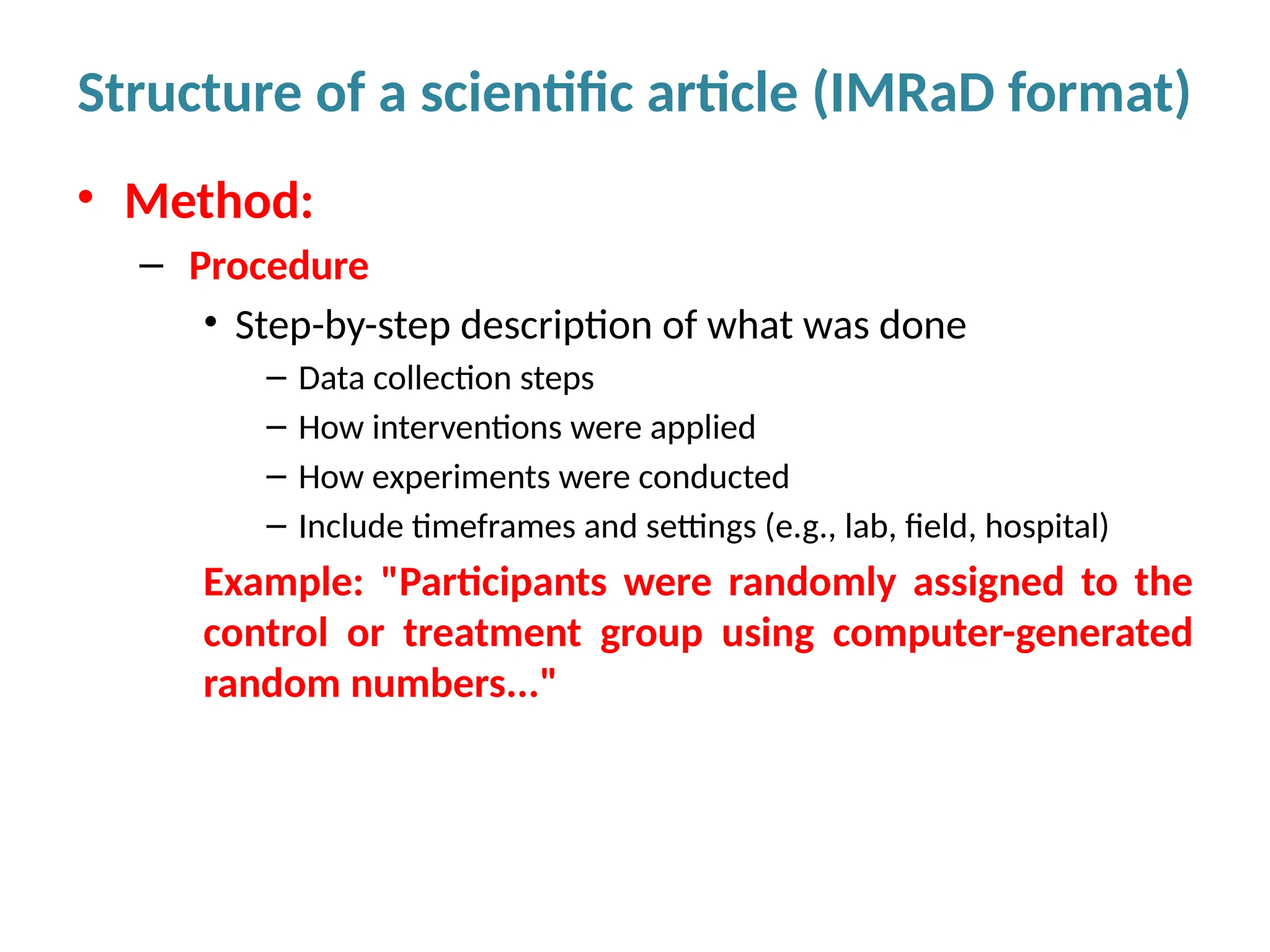
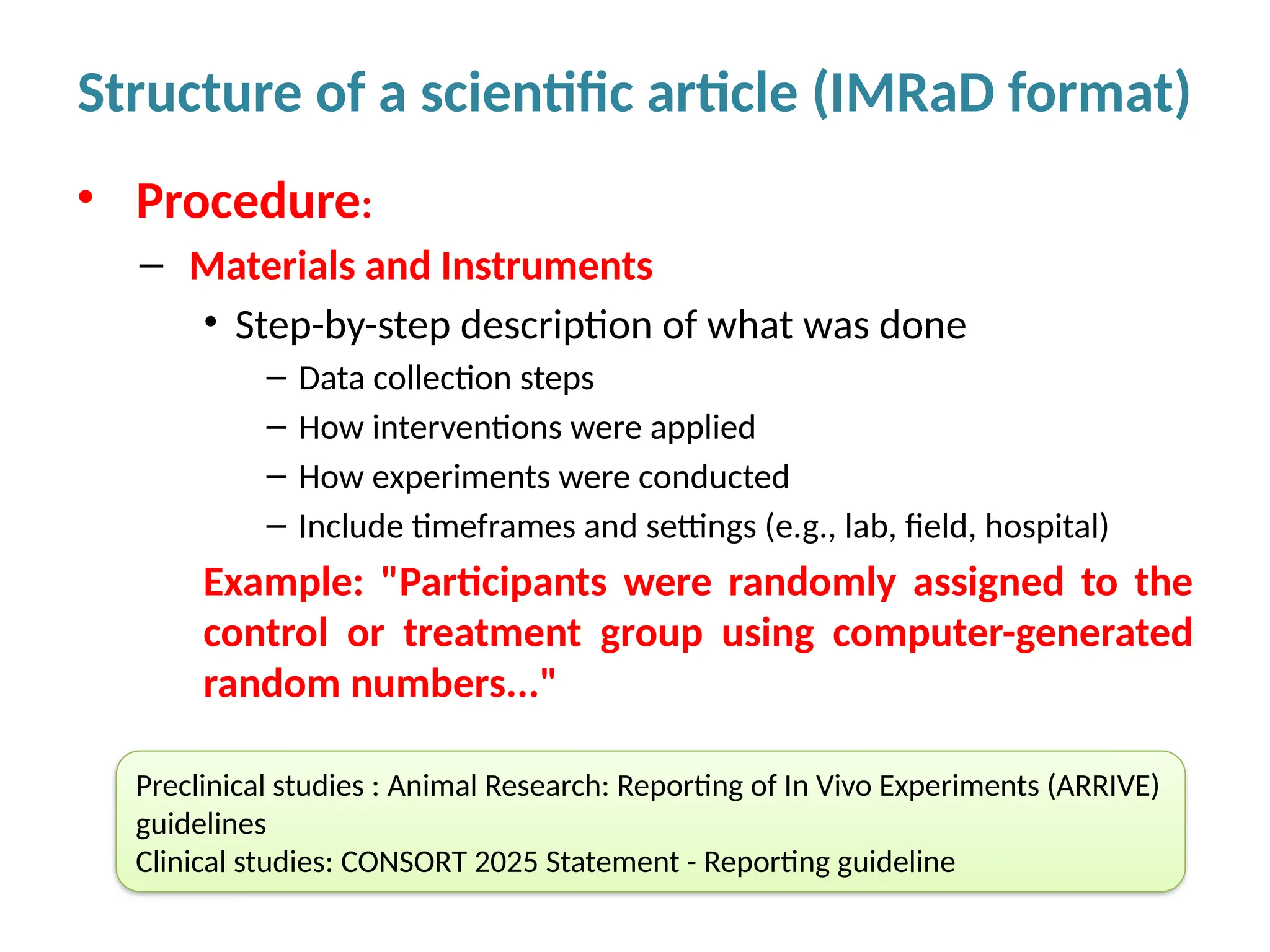
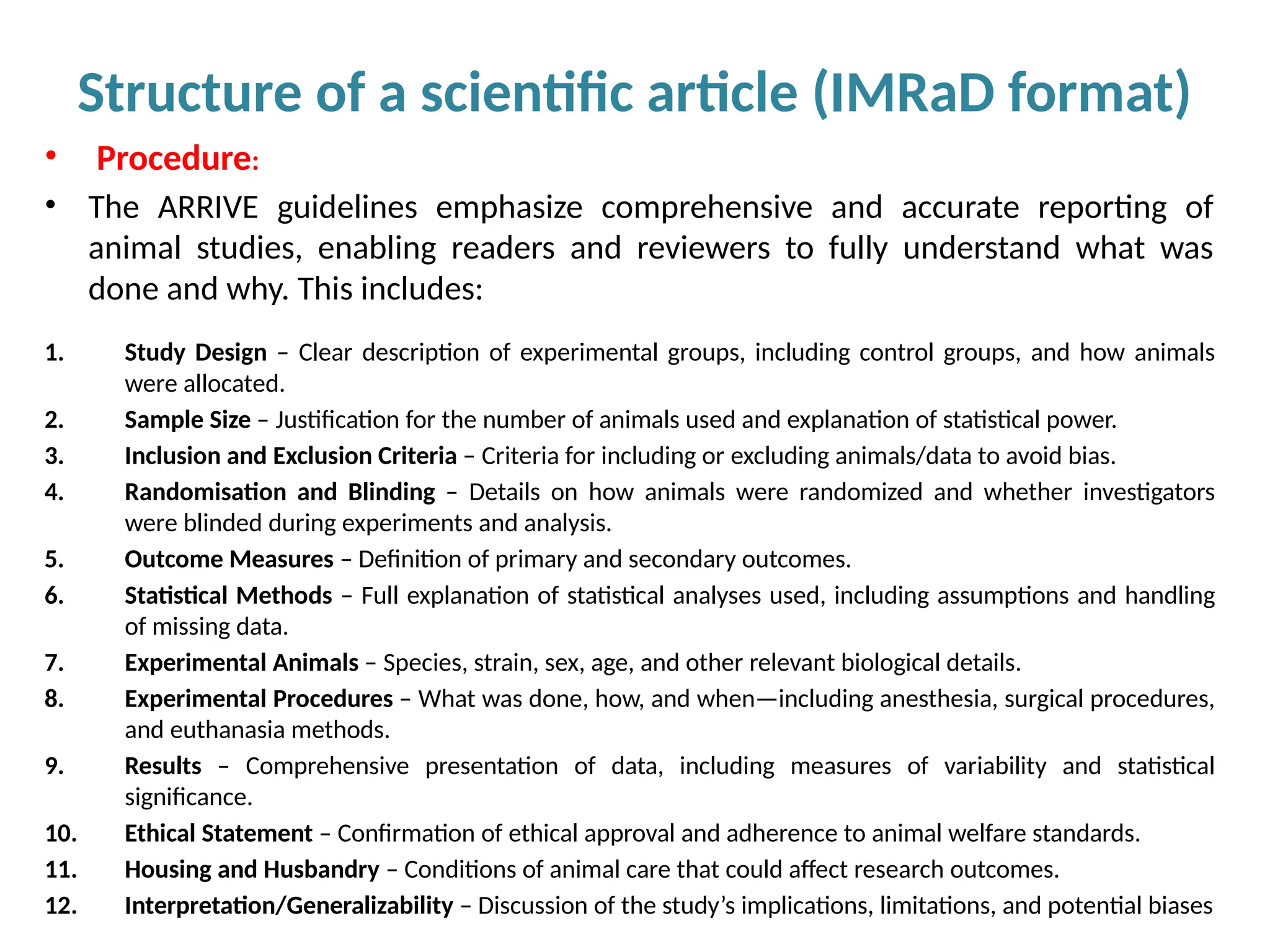
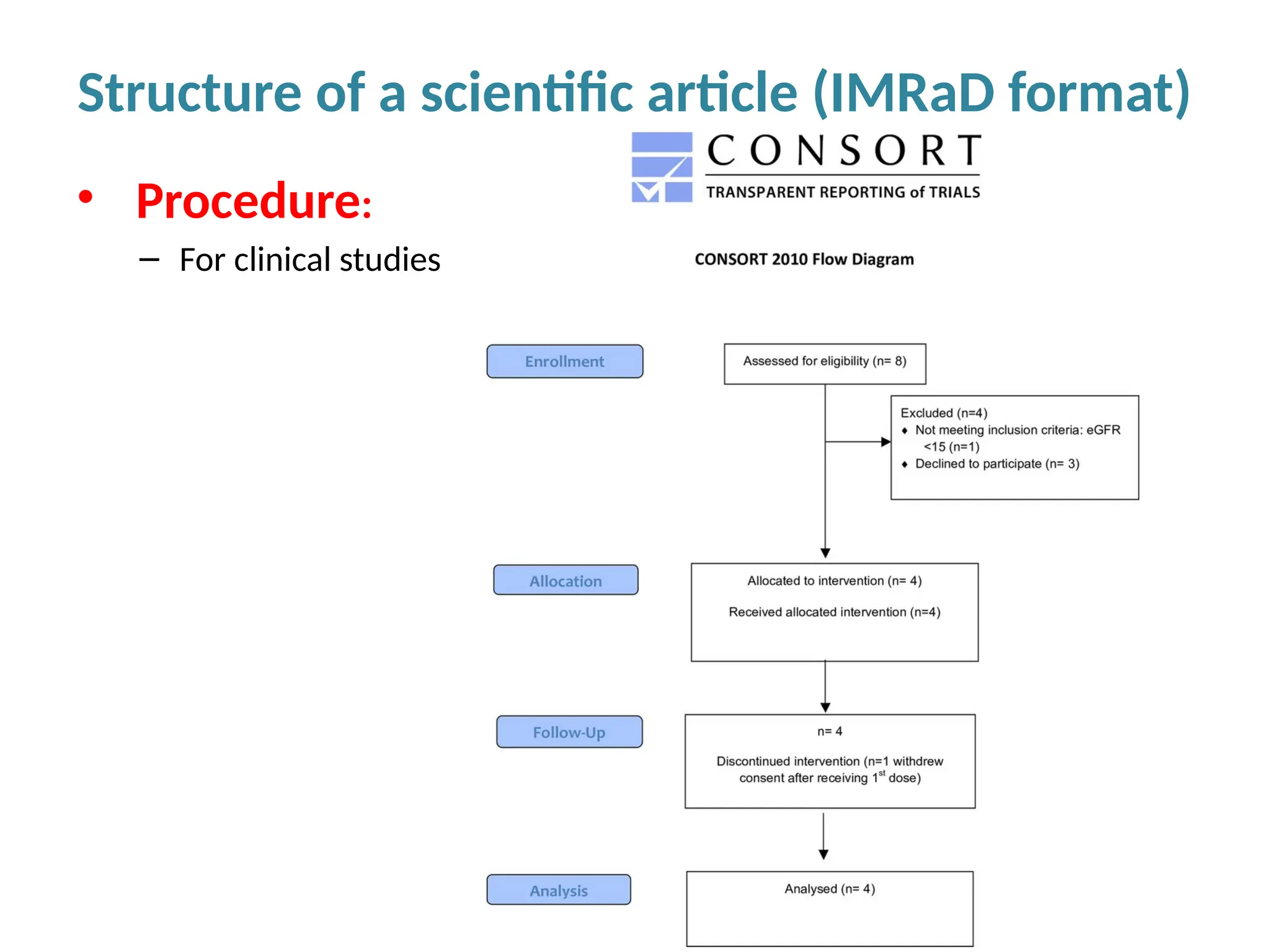
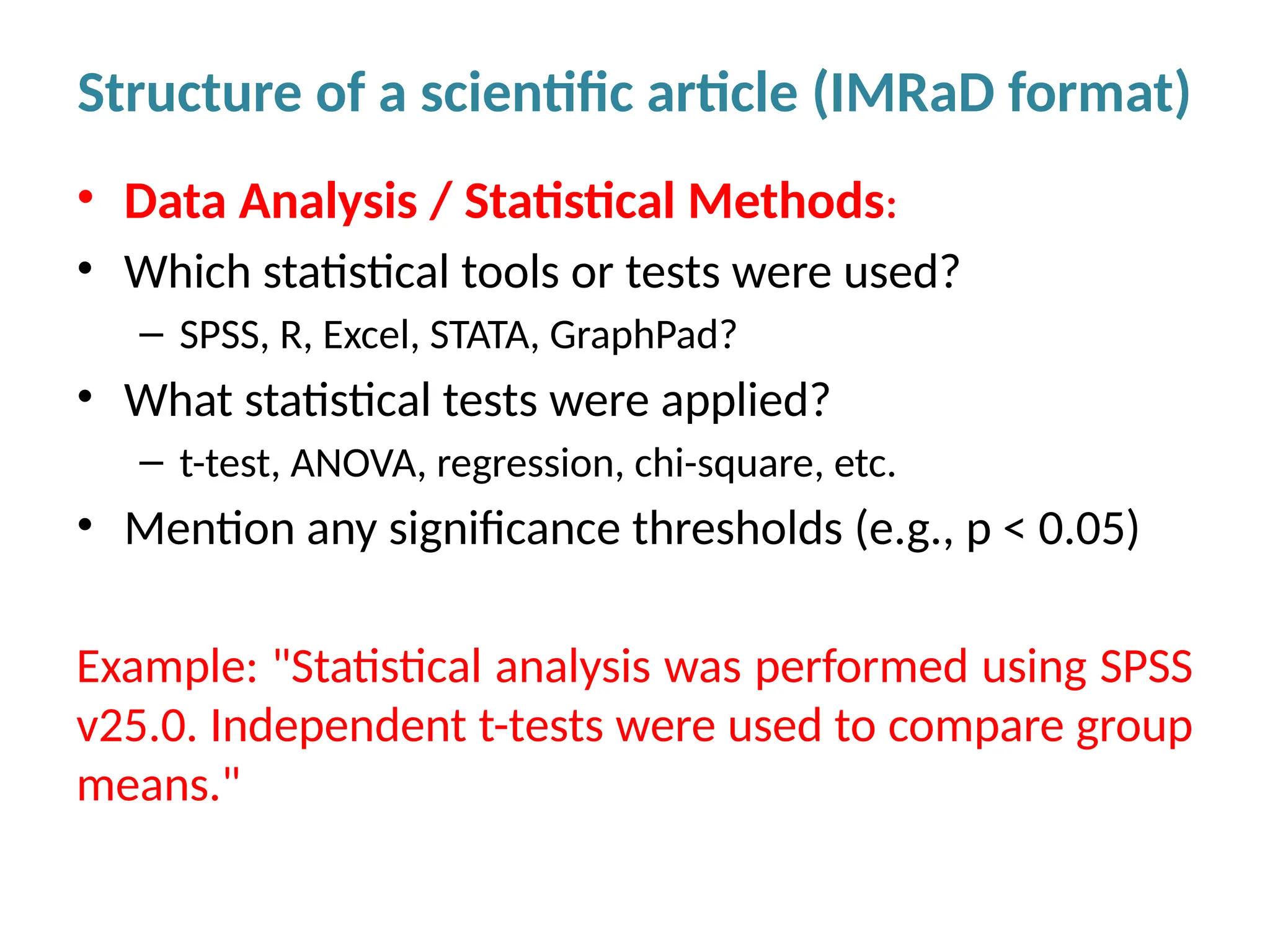
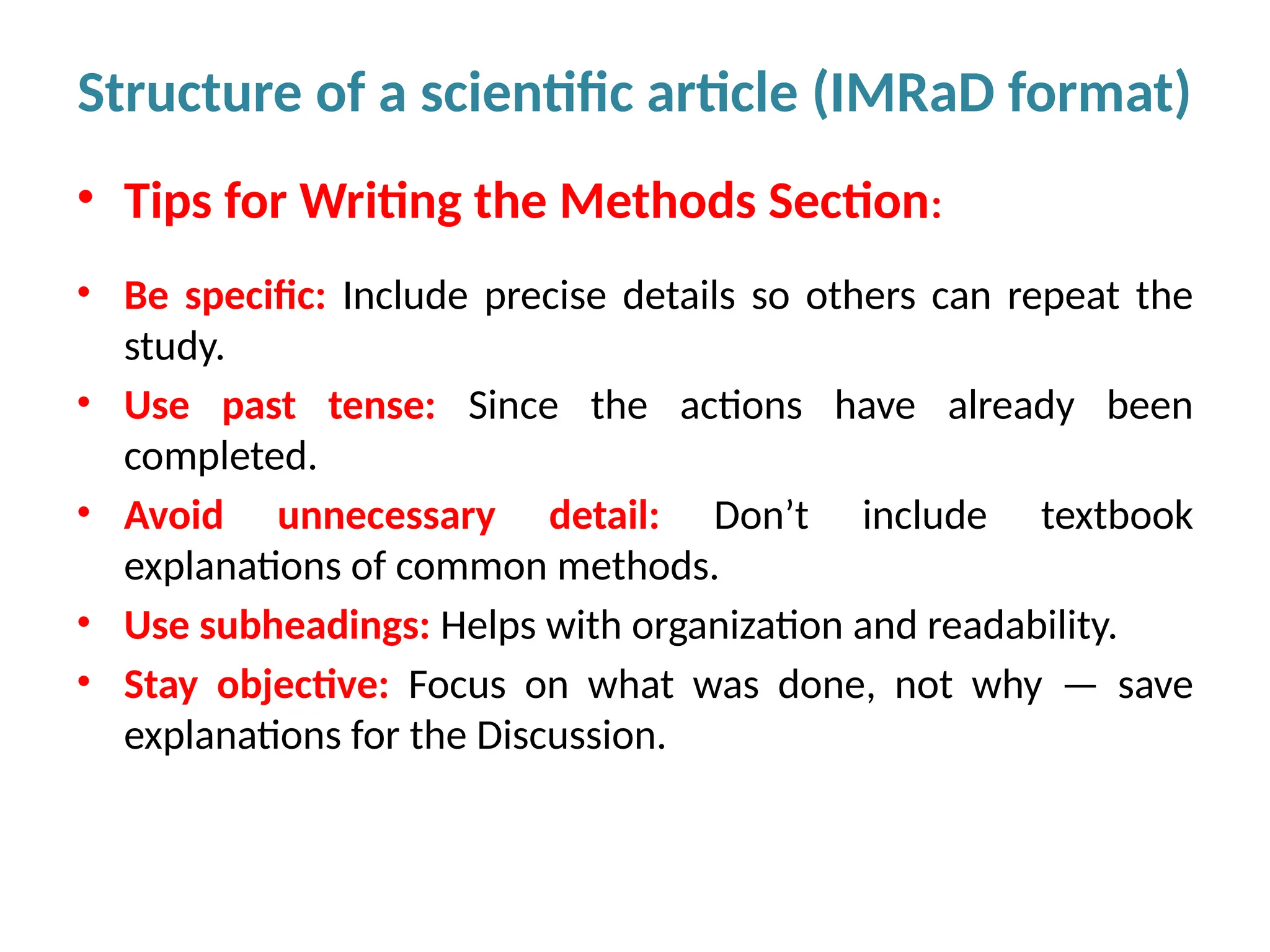
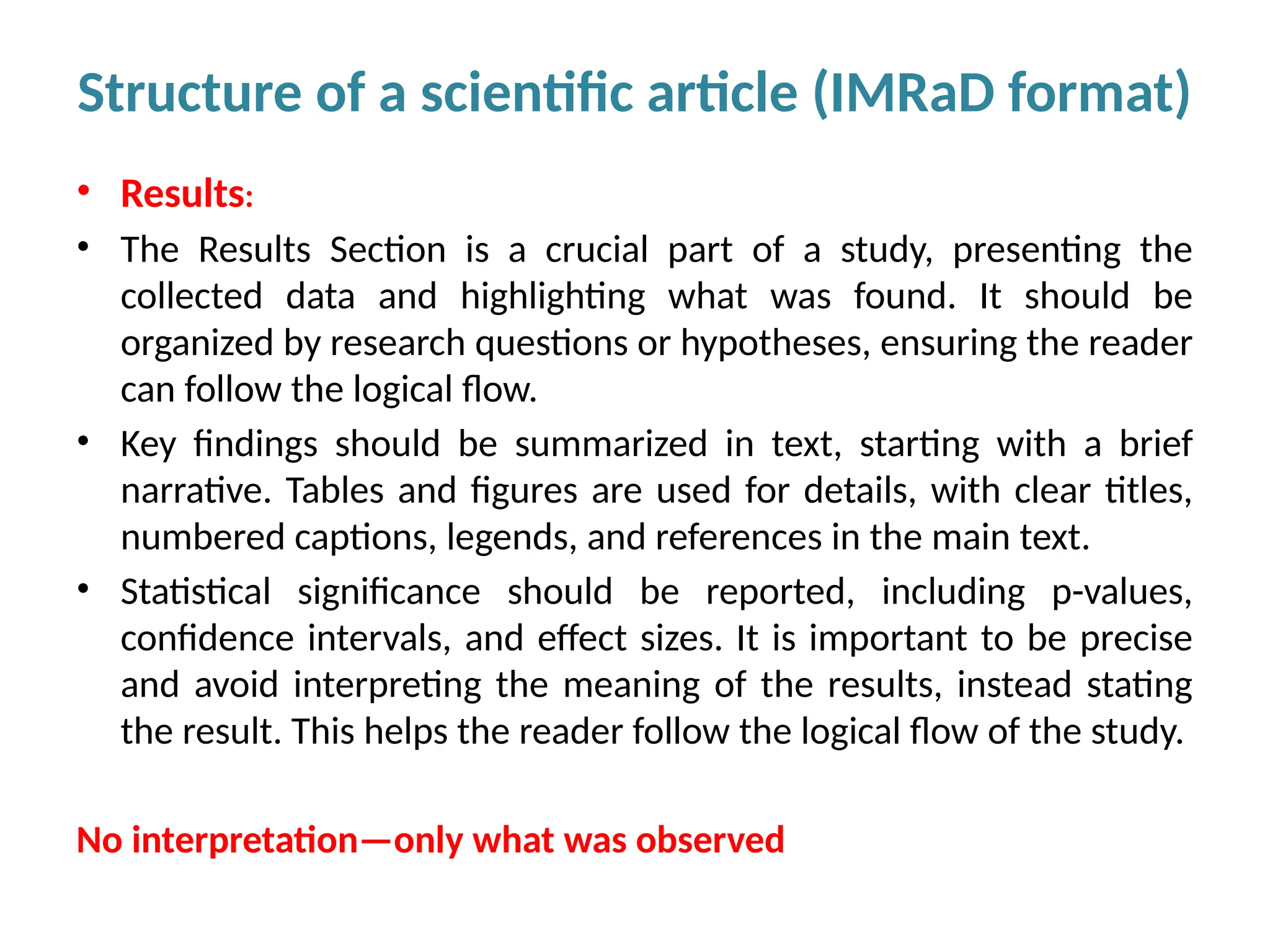
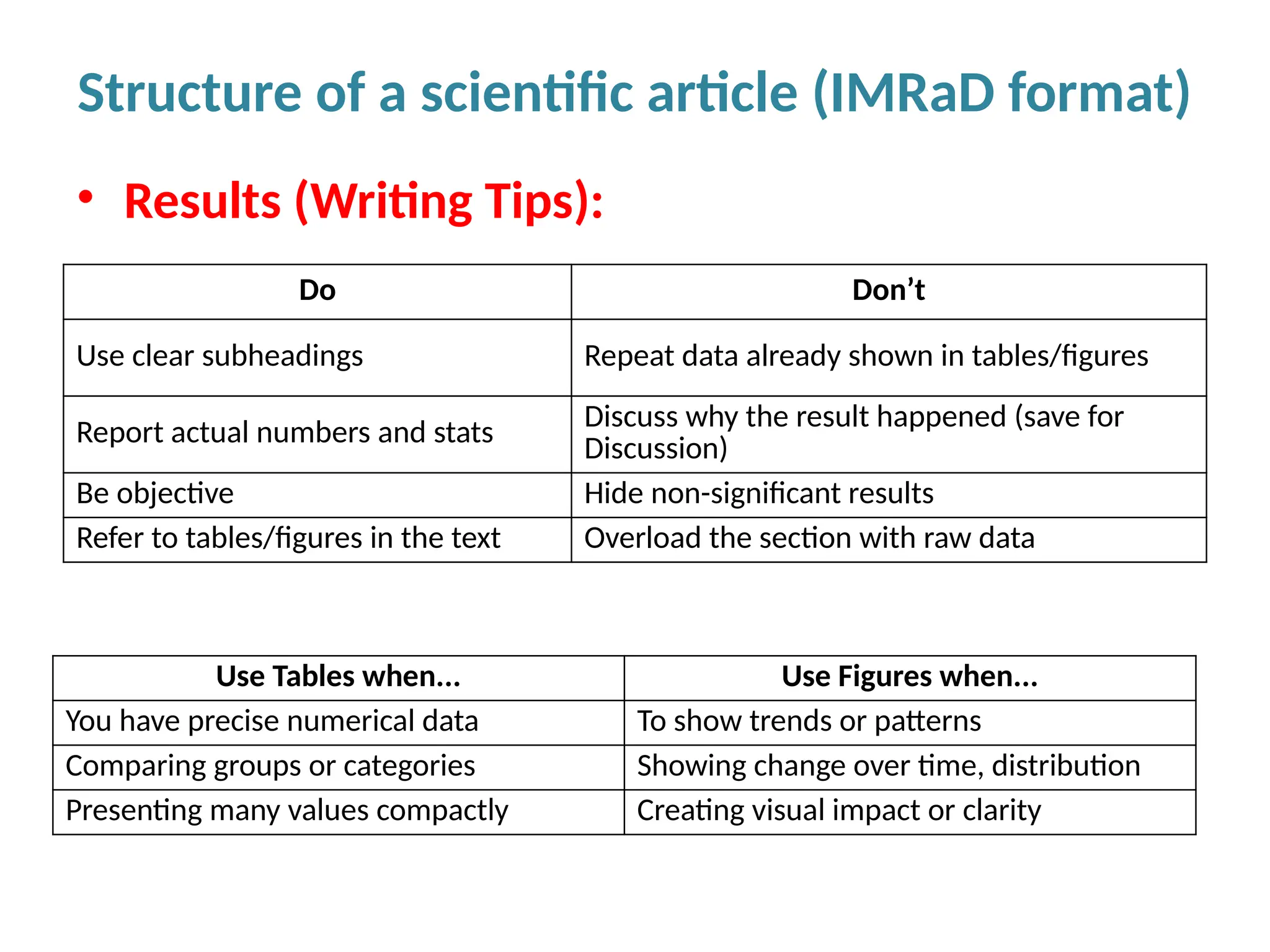
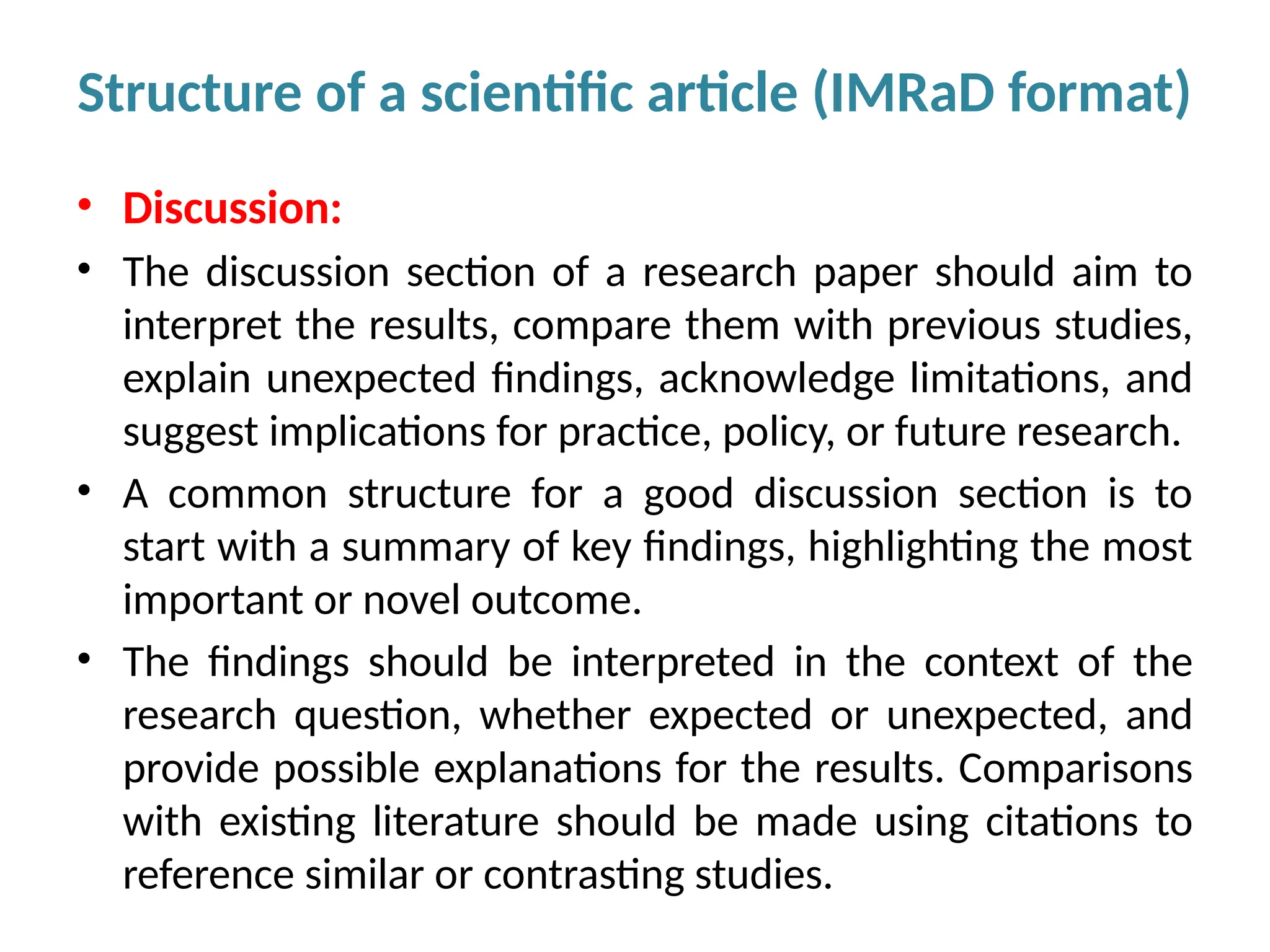
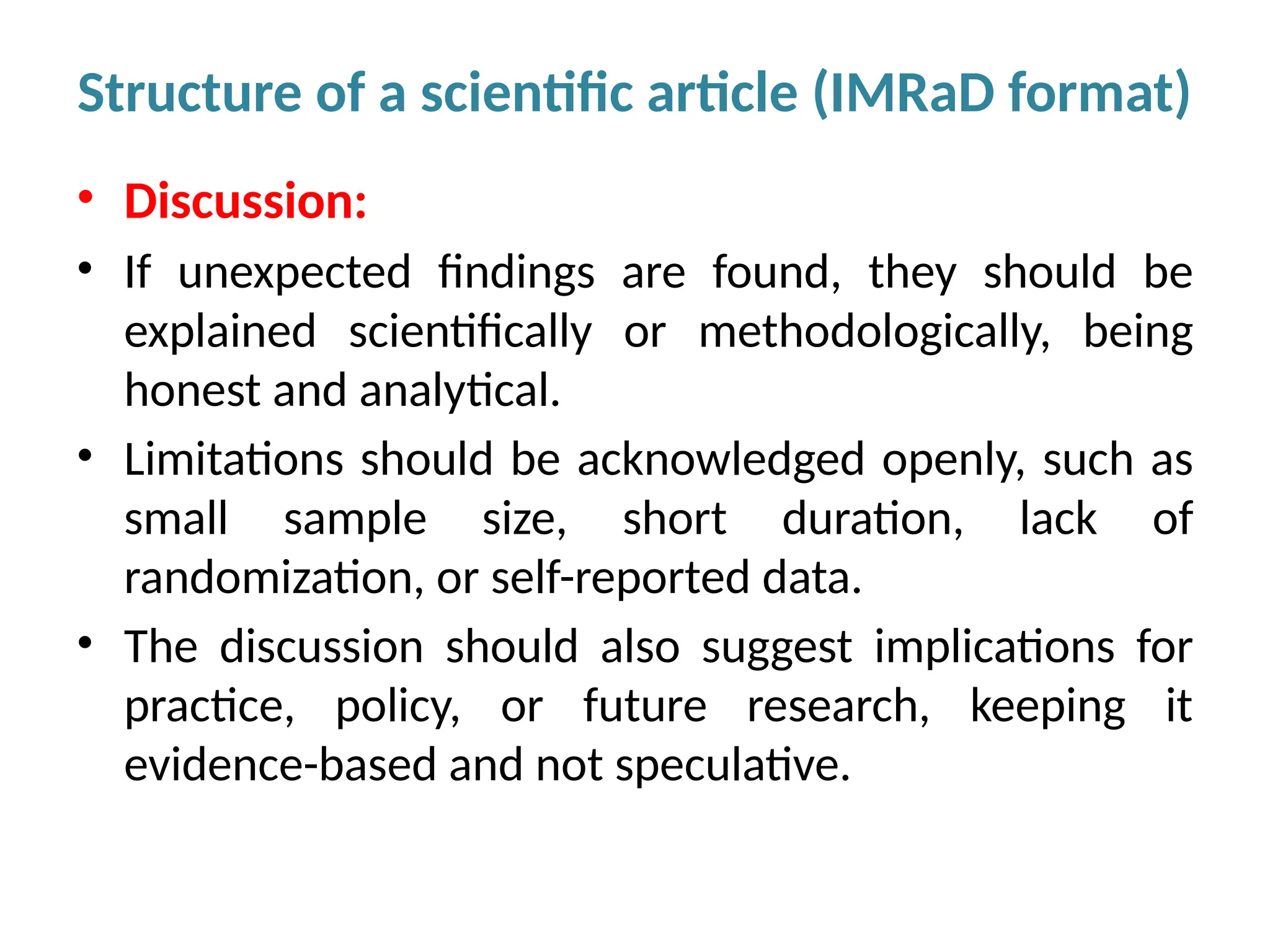
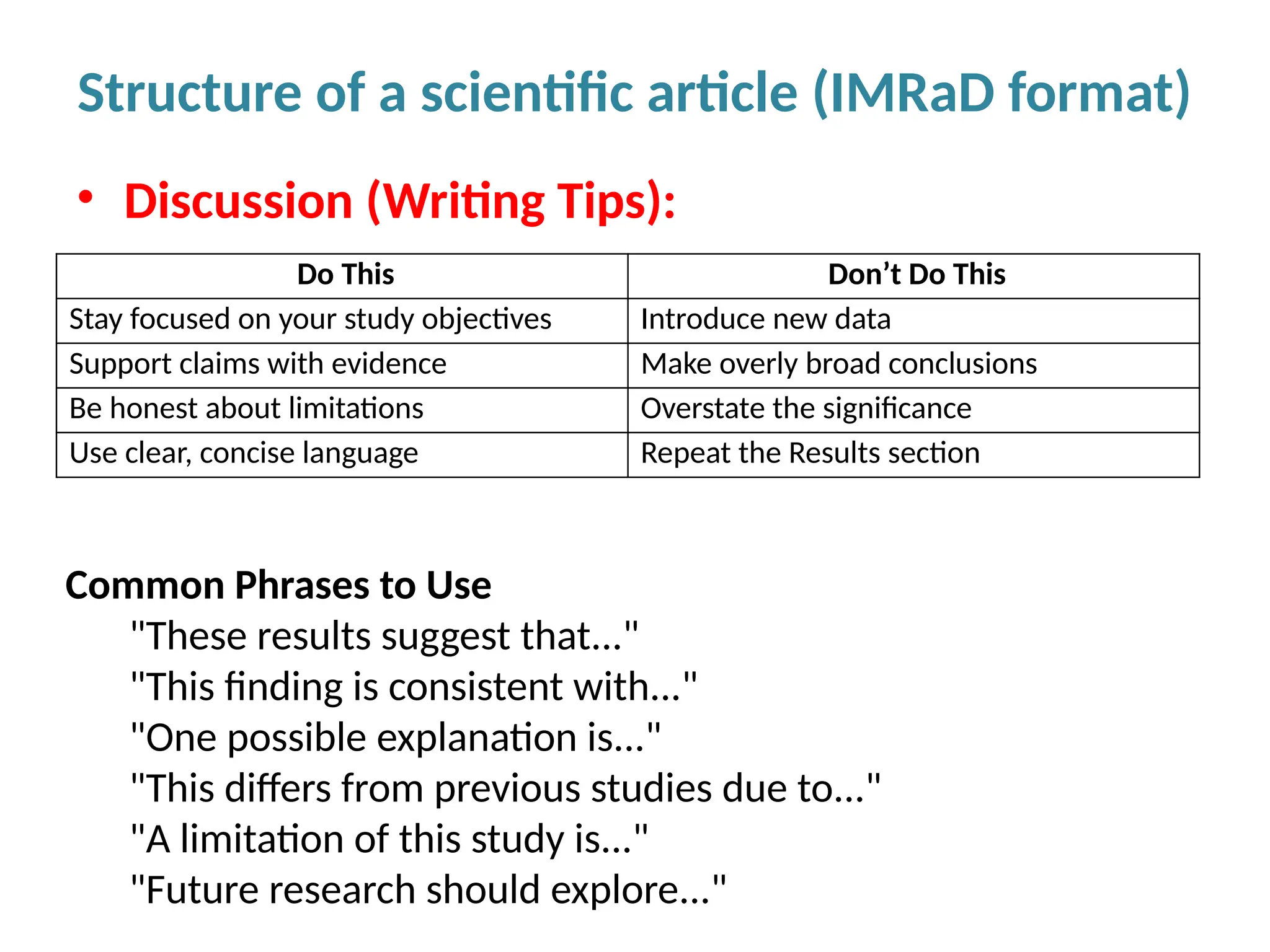
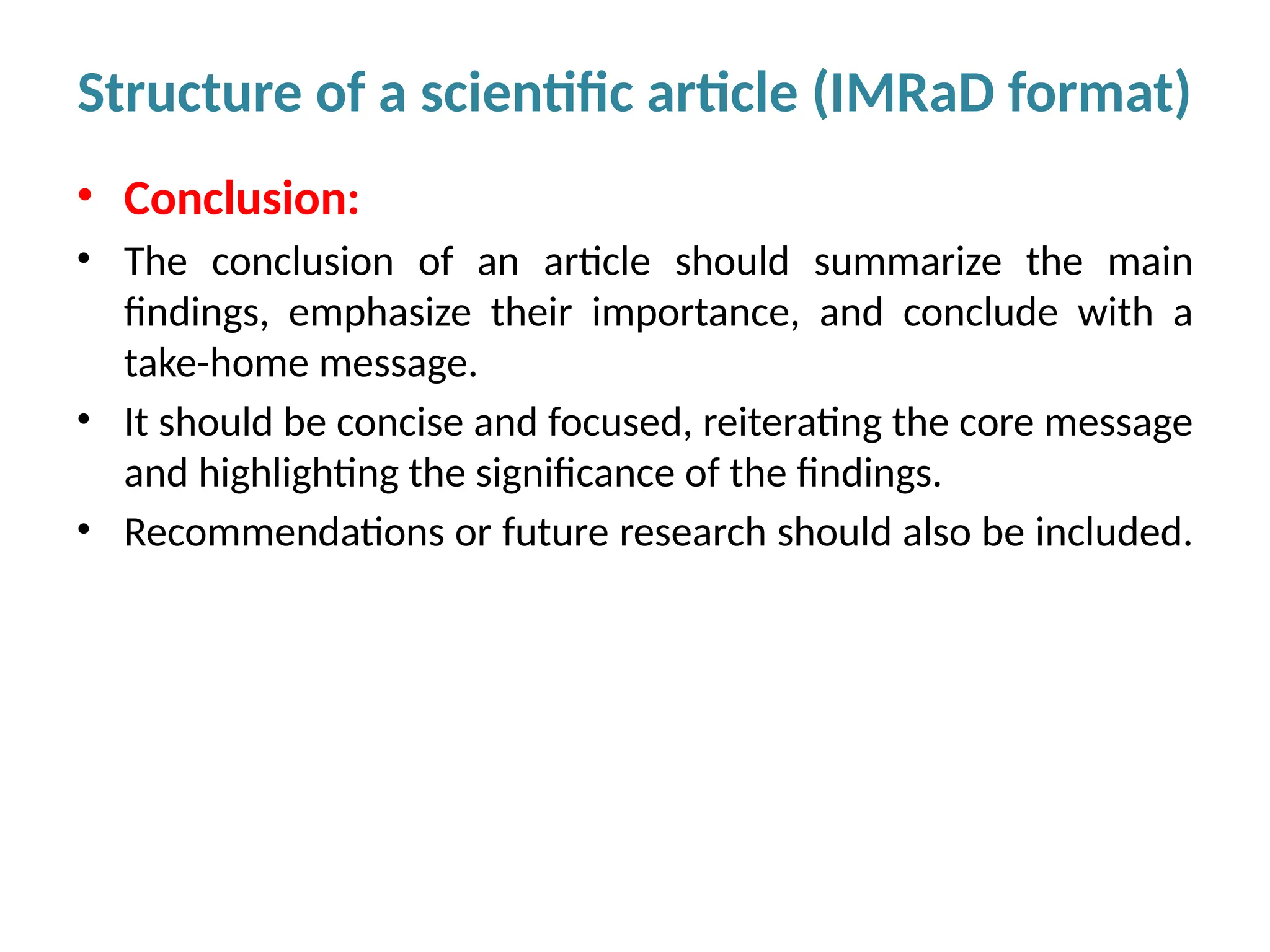
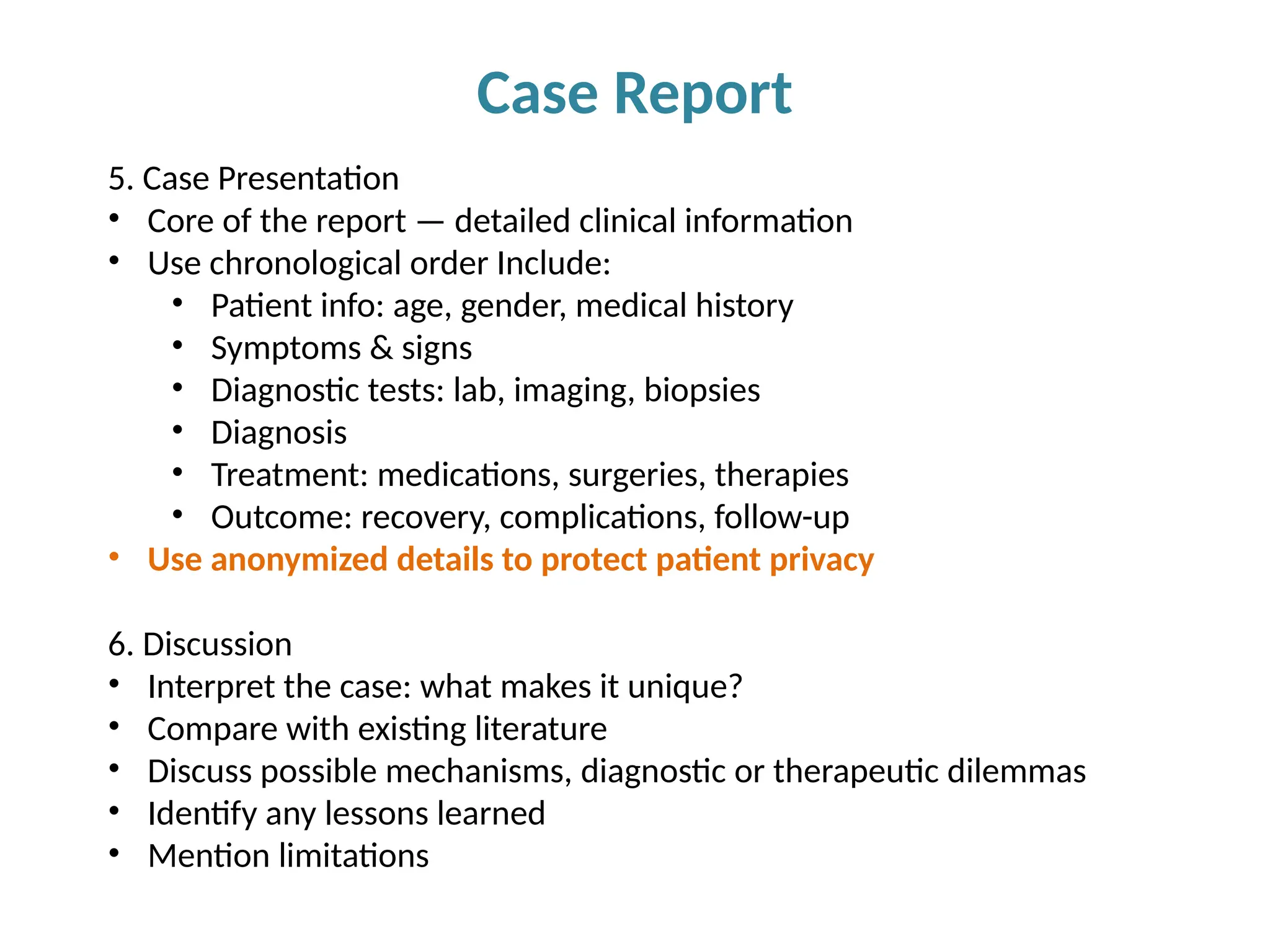
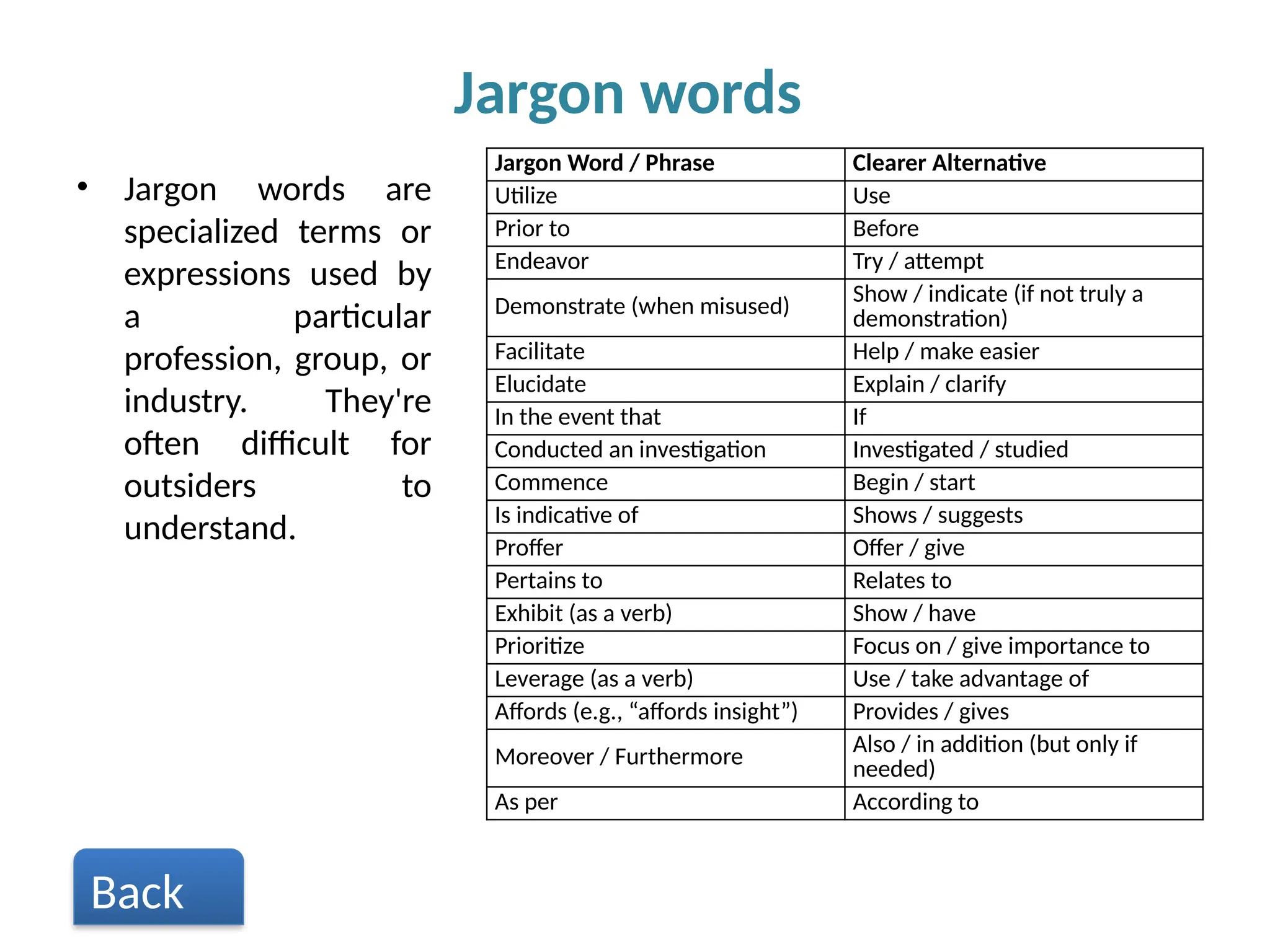
Writing effective scientific articles refers to the process of clearly, accurately, and persuasively communicating scientific research findings in a structured written format. This involves presenting original data or analysis, using a logical flow, adhering to discipline-specific conventions, and ensuring the article is understandable and valuable to the intended audience (e.g., researchers, professionals, or policy-makers). An effective scientific article typically includes sections such as the abstract, introduction, methods, results, discussion, and references, and follows ethical standards in reporting and citation.
![Writing Effective Scientific Articles
Dr. S. Parasuraman, M.Pharm., Ph.D.,
Snr. Associate Professor & Unit Head
Pharmacology, Toxicology and Basic Health Sciences [PTBHS] Unit
Faculty of Pharmacy
AIMST University
Bedong 08100, Malaysia](https://image.slidesharecdn.com/writingeffectivescientificarticles-250729082221-a69ce58a/75/Writing-Effective-Scientific-Articles-1-2048.jpg)