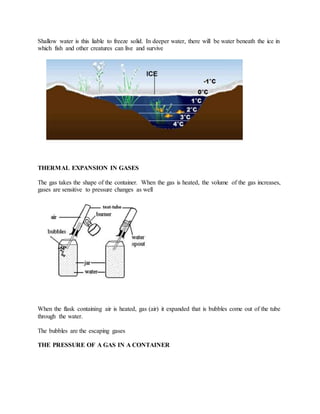
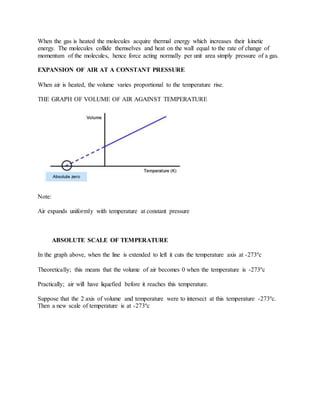
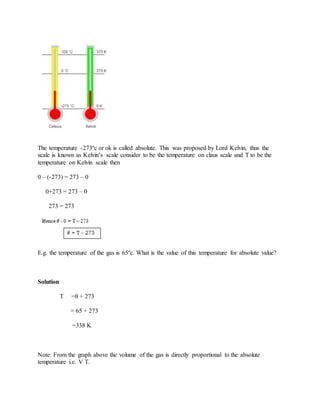
Thermal expansion is the increase in size of materials when they are heated. Metals, liquids, and gases all expand with increasing temperature. The amount of expansion depends on the material and how much the temperature changes. Bimetallic strips, which use two dissimilar metals welded together, bend when heated or cooled due to the different expansion rates of the metals. Liquids expand and decrease in density with increasing temperature. Water is unusual in that it contracts and reaches maximum density at 4°C. Gases greatly increase in volume with rising temperature at constant pressure.





![= 0.000170
3. A brick 30cm x 18cm x 10 cm is at 20ºC. If it is heated to a temperature of 50ºC, what will be
its new dimension (the coefficient of linear expansion of concrete is 1.2 x 10-5 k-)
Solution:
CASE I
New length L
L1 = L0 [1+ ∝(θ1 – θ0)]
= 30 [1+ 0.000012 (50-20)]](https://image.slidesharecdn.com/thermalexpansion-220130230510/85/Thermal-expansion-6-320.jpg)
![= 30 [1+0.000012 x 30]
= 30 [1+ 0.00036]
= 30 x 1.00036
: . L1 = 30.0108cm
CASE II
New width, w1
w1 = w0 [1+ ∝(θ1 – θ0)]
= 18 [1+ 0.000017 x 30]
= 18 [1+0.00036]
= 18 x 1.00036
: . w1 = 18.00648cm
CASE III
New height, h
h1 = h0 [1+ ∝(θ1 – θ0)]
= 10[1+0.000012 (30)]
= 10(1+0.00036)
= 10 x 1.00036
: . h1 = 10.0036cm
4. An iron plate at 20ºC as a whole of radius 8.92mm in the centre. An iron rivet with the radius
of 8.95mm at 20ºC is to be inserted into the hole. To what temperature most the plate will be
heated for the rivet to fit into the whole (linear expansively of iron is 1.24 x 10-5 k-1)](https://image.slidesharecdn.com/thermalexpansion-220130230510/85/Thermal-expansion-7-320.jpg)




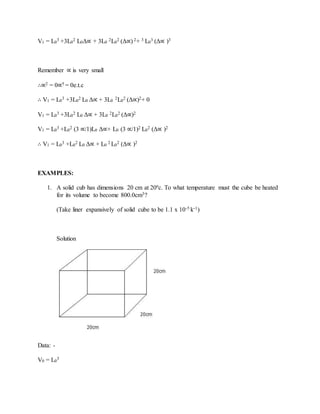
![V0 = L0 X L0 X L0
V0 = L0
3.............. (i)
When a cube is heated to temperature
V1 = (L0 +ΔL) (L0 +ΔL) (L0 +ΔL)
V1 = [L02 +2L0 ΔL (ΔL) 2]
V1 = L0
3 +2L0
2 ΔL+ 3L0 (ΔL) 2 + (ΔL) 3
V1 = L0
3 +3L0
2 ΔL+ 3L0 (ΔL)2 + (ΔL)3
But; ΔL = L0 Δ∝
V1 = L0
3 +3L0
2 (L0Δ∝) + 3L0 (2L0
2 (Δ∝)2)+ L3
0
3(Δ∝ )3](https://image.slidesharecdn.com/thermalexpansion-220130230510/85/Thermal-expansion-12-320.jpg)