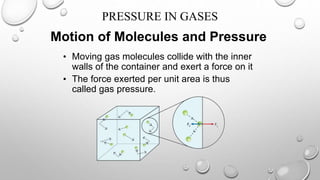
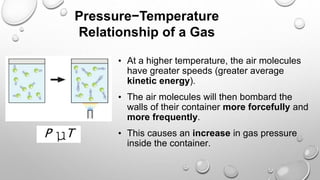
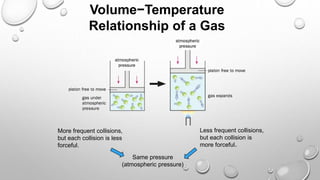
The document discusses the relationships between pressure, temperature, and volume in gases. It explains that gas pressure increases with temperature because the faster moving gas molecules collide with the container walls more frequently and forcefully. It also explains that gas volume increases with temperature as the molecules move further apart while maintaining a constant pressure through less frequent but more forceful collisions. Finally, it notes that gas pressure increases with decreasing volume, as molecules collide with the walls more often in a smaller space.