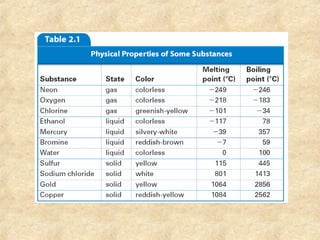
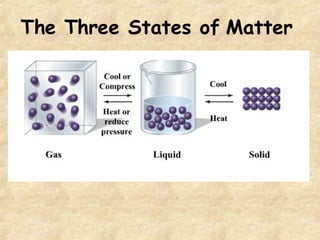
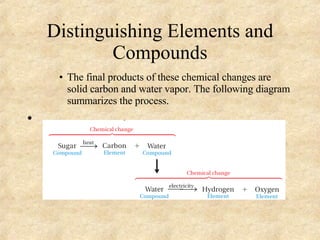
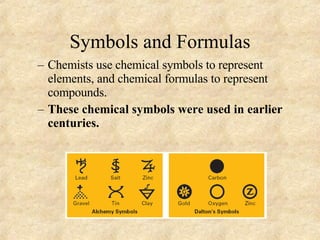
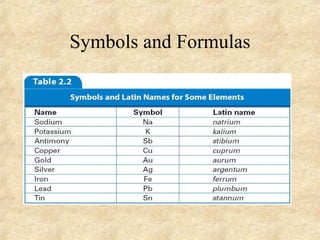
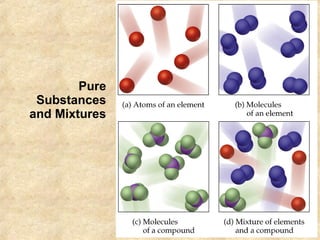
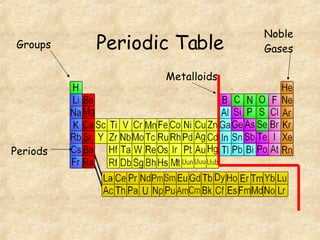
The document discusses various topics related to matter and chemical changes. It defines elements and compounds, explains that compounds can undergo chemical changes while elements cannot, and states that the law of conservation of mass means the mass of reactants equals the mass of products in a chemical reaction. It also distinguishes physical and chemical changes, noting that only chemical changes alter the composition of matter.