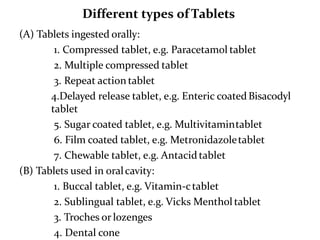
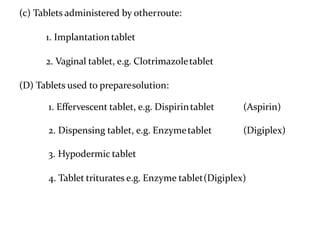
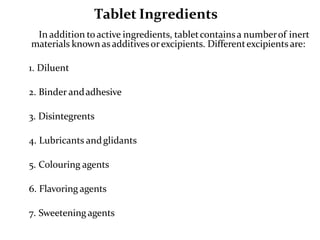
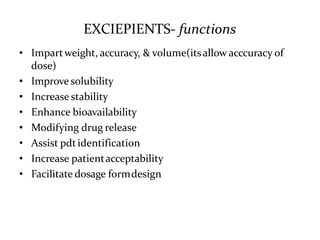
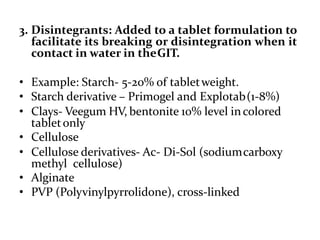
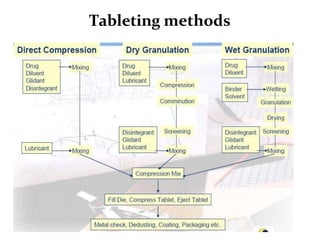
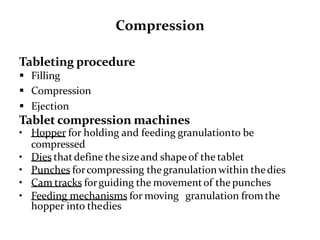
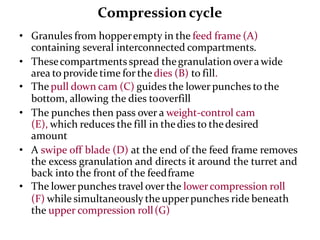
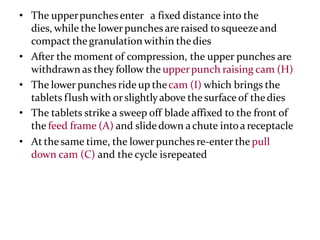
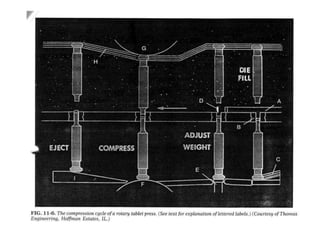
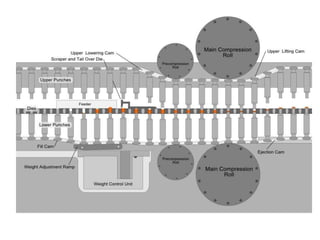
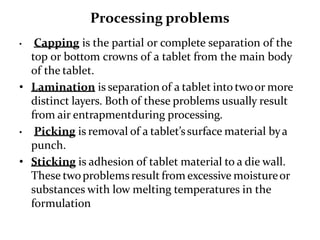
The document provides information about tablets, including their definition, advantages, disadvantages, types, ingredients, and manufacturing methods. It discusses that tablets are a solid dosage form containing medicaments that are manufactured by compressing powders into a solid mass using compression. The main methods of tablet manufacturing discussed are direct compression, dry granulation, wet granulation, and roller compaction. Excipients like diluents, binders, disintegrants, and lubricants are added to optimize the tableting process and tablet properties.