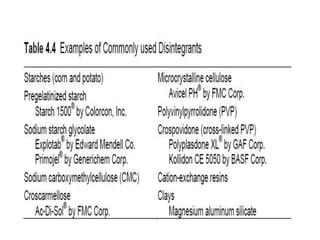
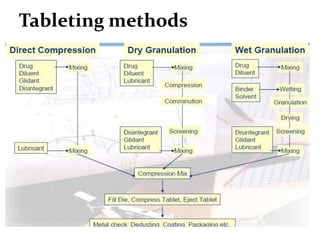
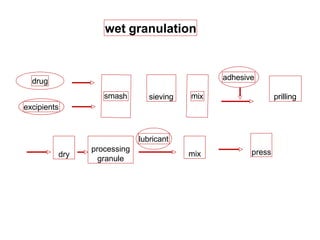
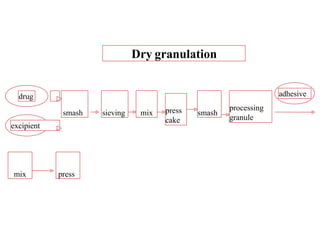
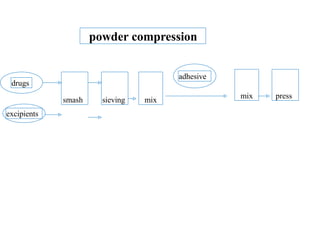
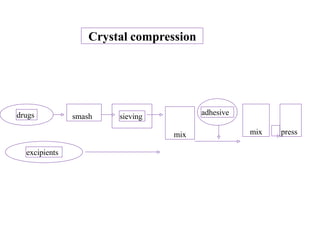
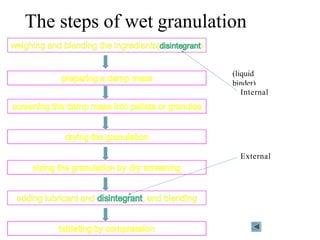
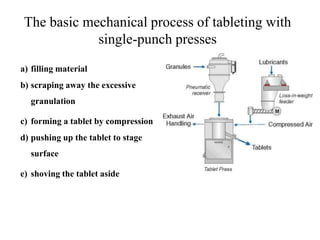
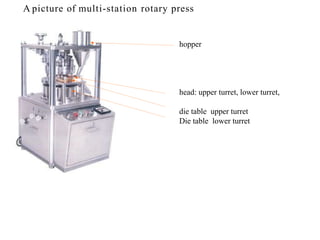
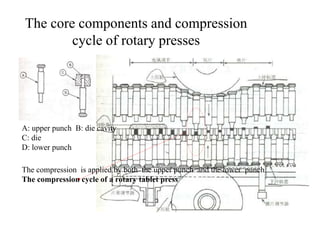
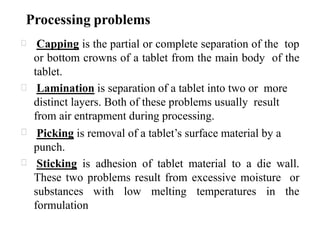
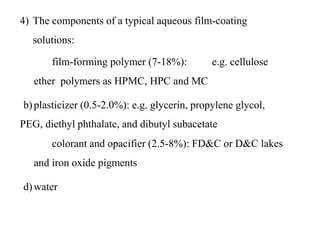
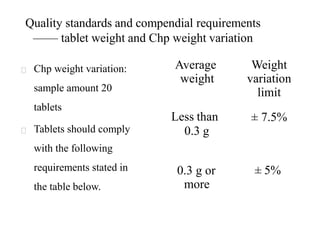
This presentation discusses tablet formulation and manufacturing. Tablets are defined as compressed solid dosage forms containing active ingredients with or without excipients. Tablets offer advantages for large scale production, packaging/shipping, stability, and dosage precision. Ingredients include active drugs and excipients like diluents, binders, disintegrants, lubricants. Tableting methods include direct compression, wet and dry granulation. Tablet presses include single-punch and multi-station rotary presses. Process steps are filling, compression, and ejection. Common problems are capping and lamination from air entrapment.