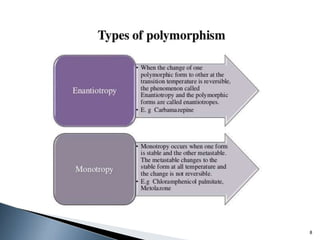
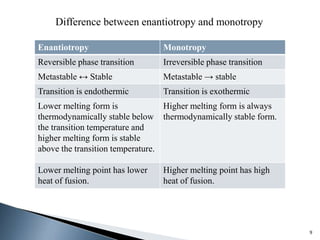
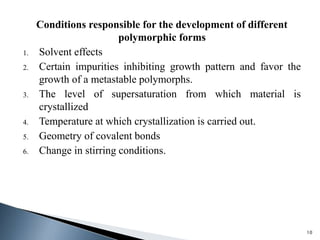
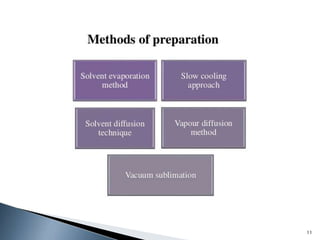
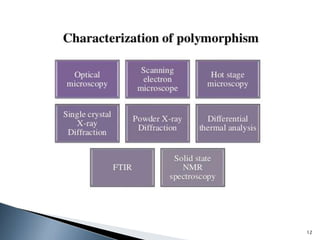
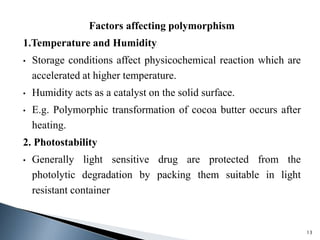
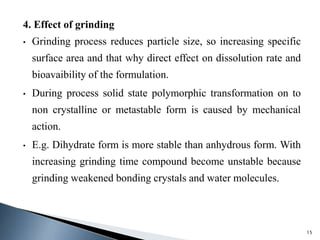
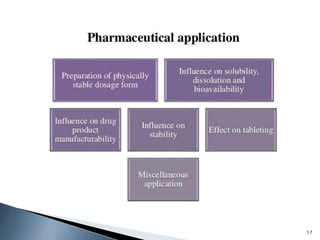
The document discusses polymorphism, the ability of a substance to exist in multiple crystalline forms with differing molecular arrangements, highlighting its significance in pharmaceuticals, agrochemicals, and other fields. It covers factors influencing polymorphism, methods of preparation, and the implications of polymorphic forms on drug development, solubility, and bioavailability. The conclusion emphasizes the importance of understanding polymorphism to make informed decisions in the progression of drug formulations.