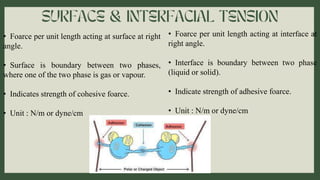
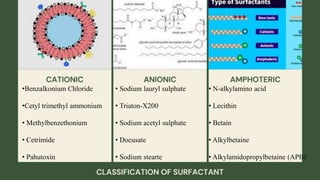
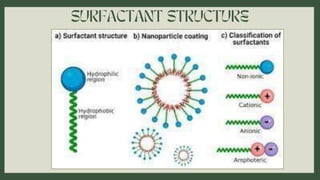
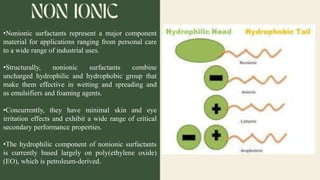
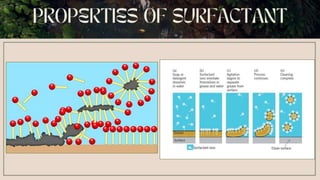
Surfactants are substances that lower surface tension and form micelles. They have both a hydrophobic tail and hydrophilic head. Surfactants are used as detergents, emulsifiers, fabric softeners, and in oil recovery. The critical micelle concentration is the minimum amount of surfactant needed to reduce surface tension and form micelles.