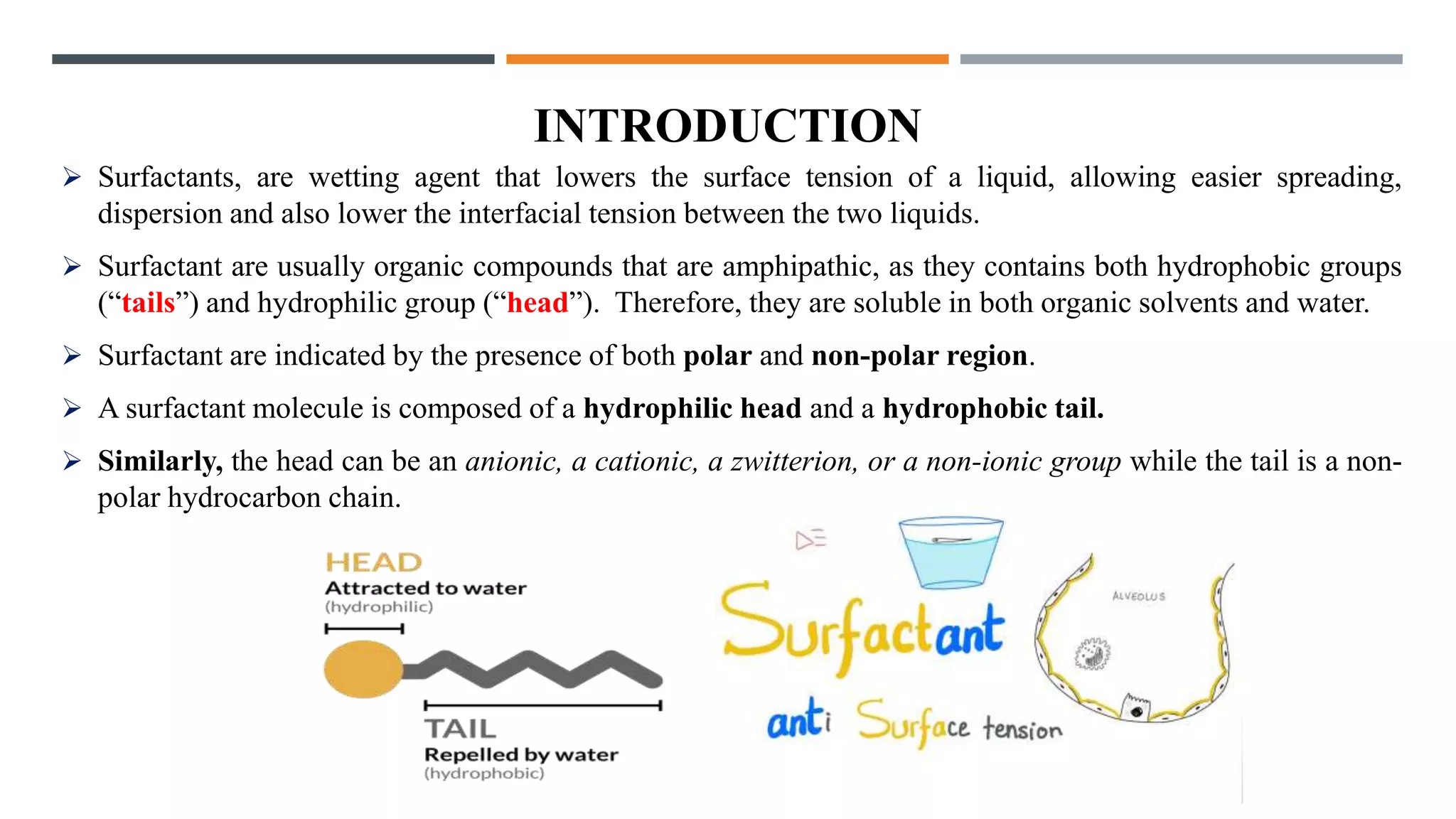
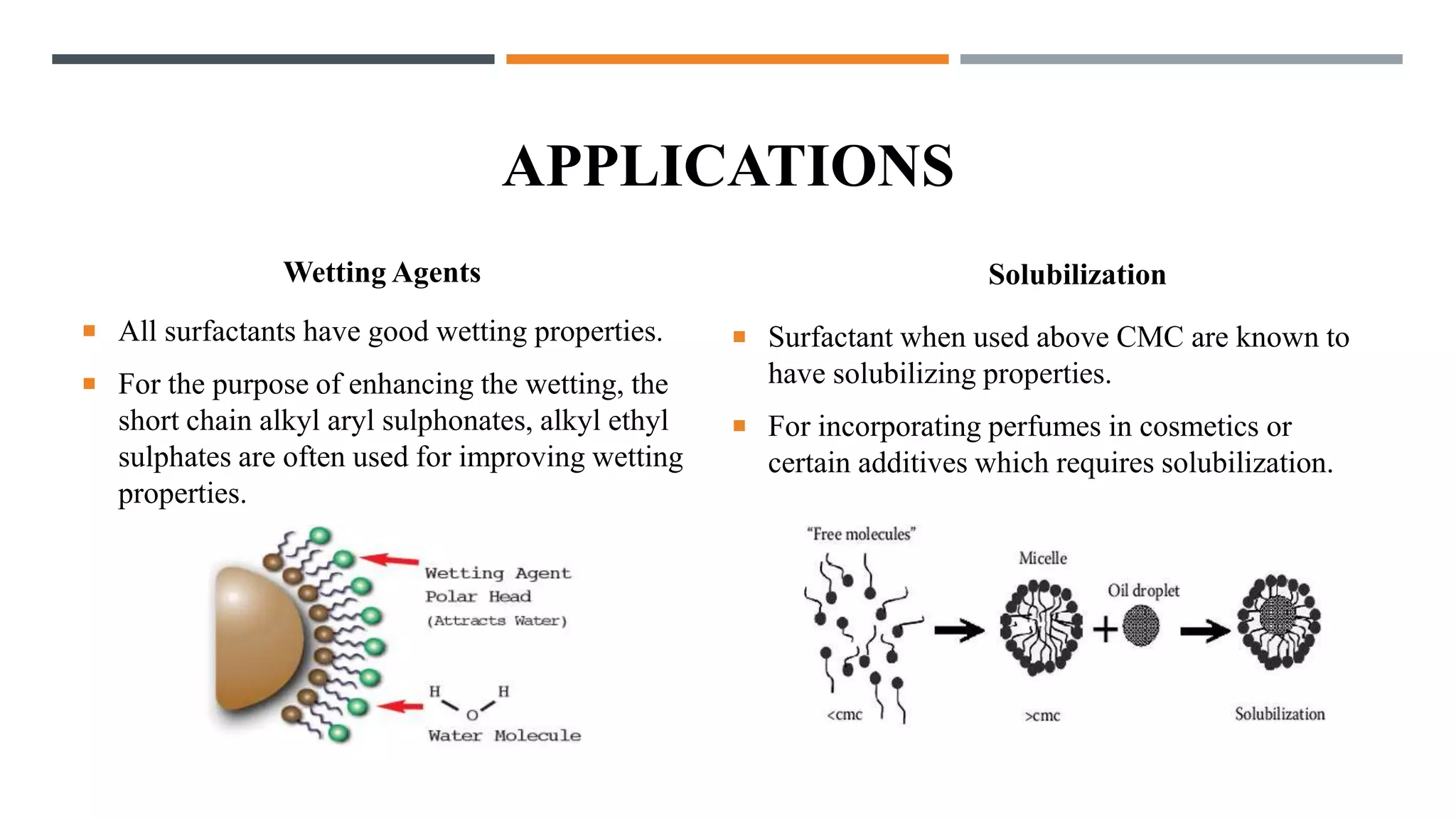
Surfactants lower the surface tension of liquids and the interfacial tension between two liquids. They are amphipathic molecules with both hydrophilic and hydrophobic regions. Surfactants are classified as anionic, cationic, non-ionic, or amphoteric based on the nature of their hydrophilic group. Anionic surfactants have a negatively charged hydrophilic group, while cationic have a positively charged group. Non-ionic surfactants have uncharged polar groups like ethylene oxide. Amphoteric surfactants can form both positive and negative surface-active ions. Surfactants are commonly used as emulsifying, foaming, cleansing, and wetting agents in cosmetics