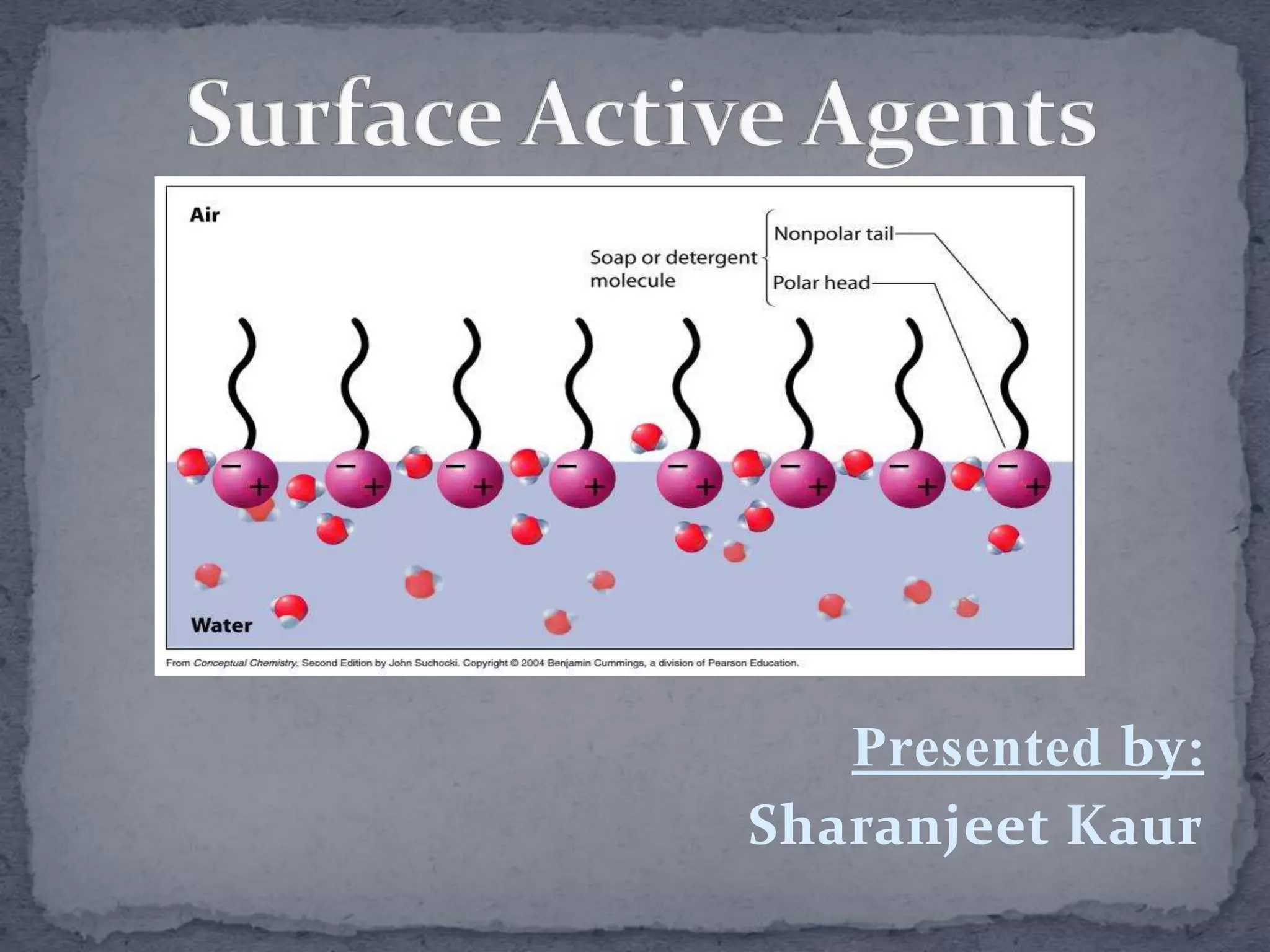
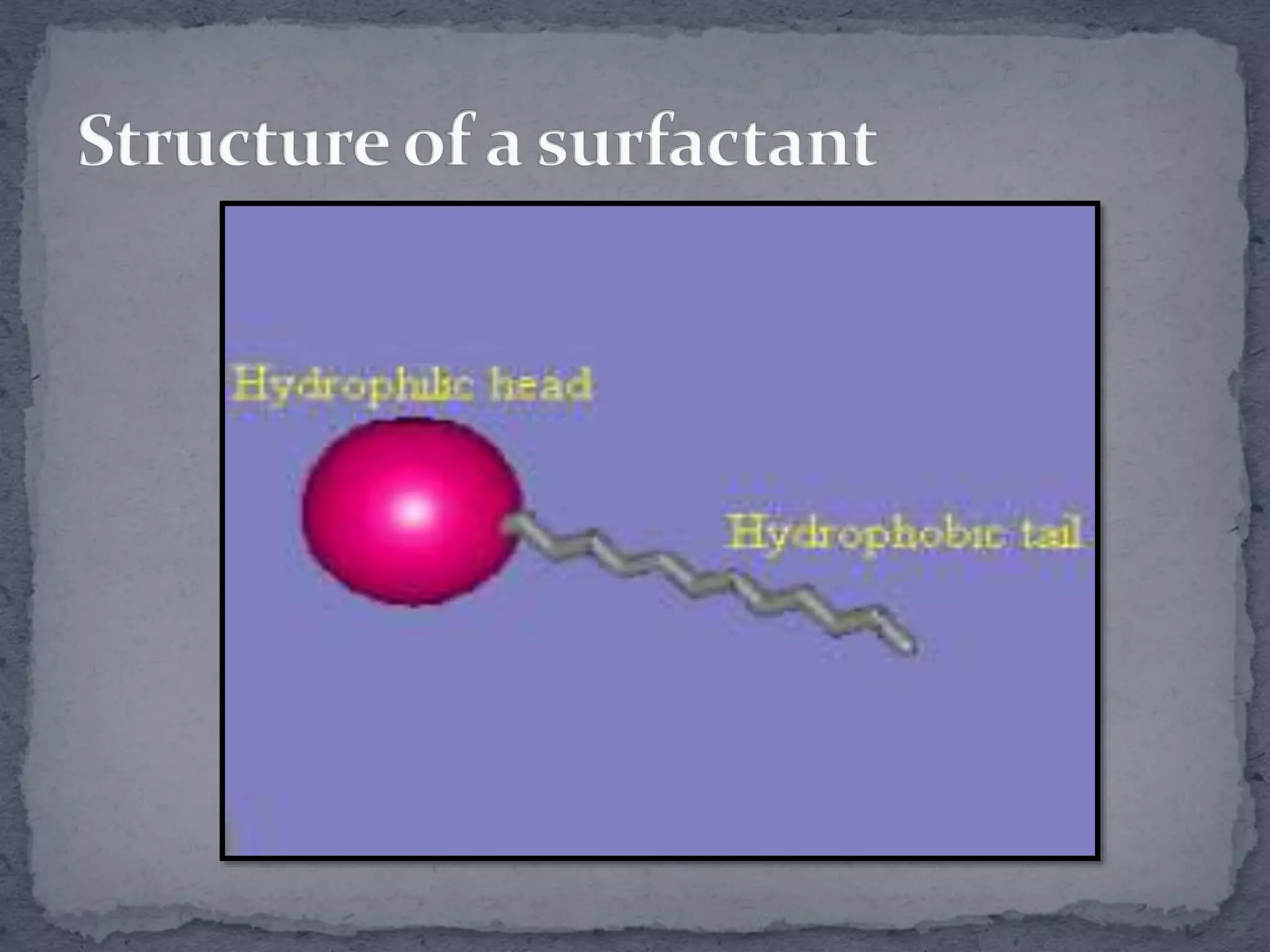
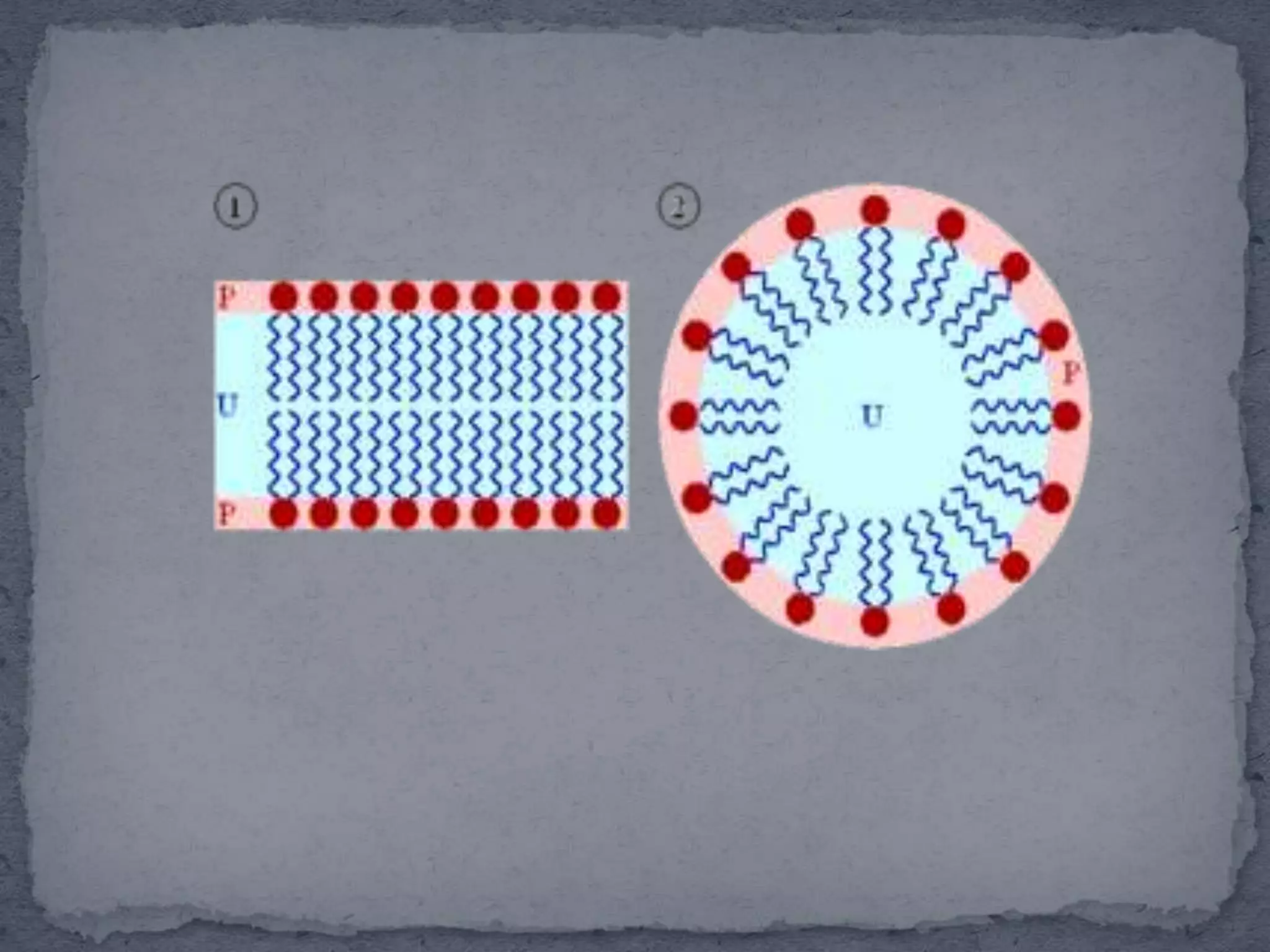
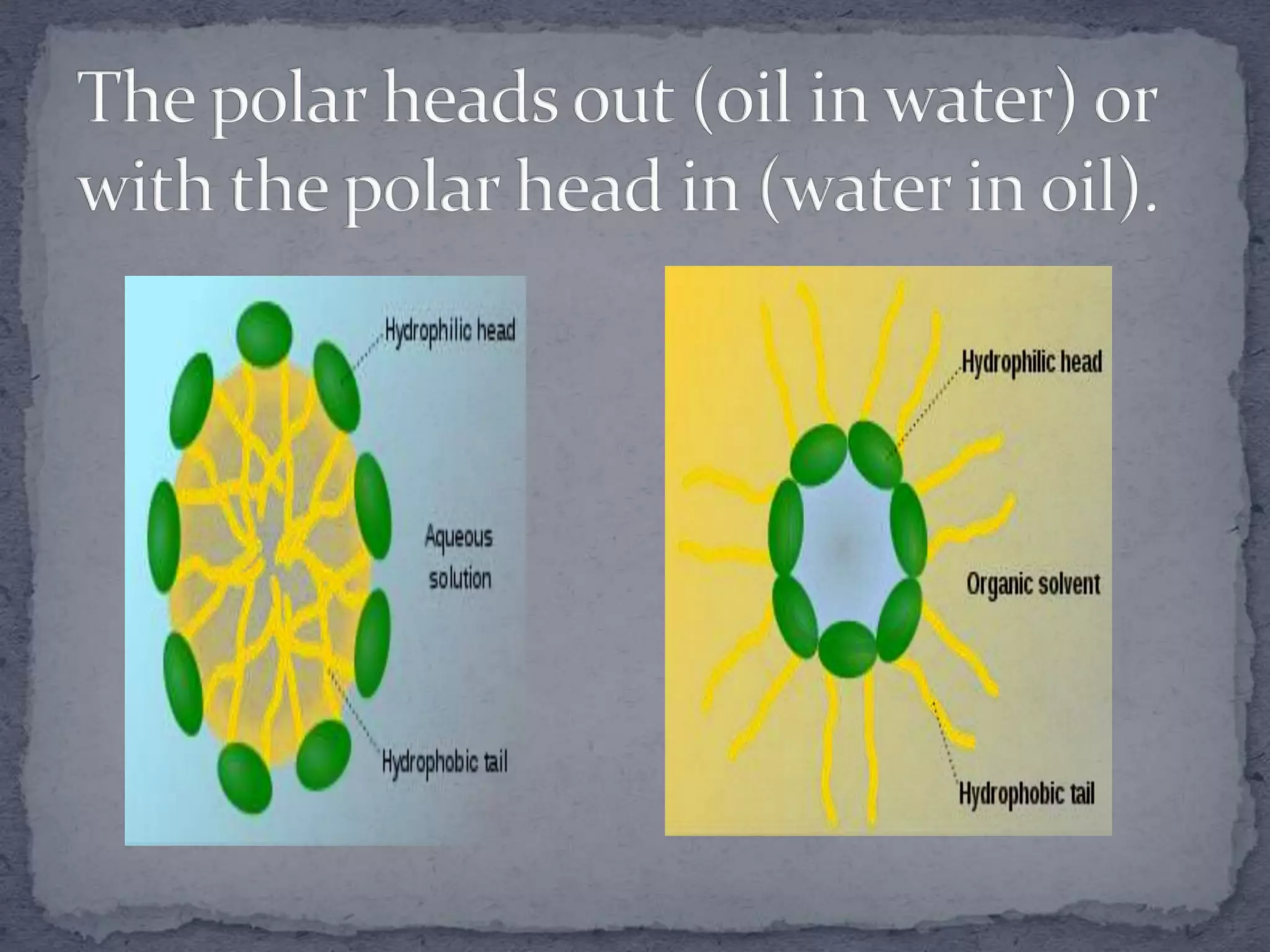
The document discusses surfactants and micelles. It defines hydrophilic, hydrophobic, lipophilic and lipophobic surfaces or liquids. It explains that surfactants are amphipathic molecules with both polar and nonpolar portions that can interact with both polar and nonpolar substances. Surfactants form spherical clusters called micelles in water, with nonpolar tails inside and polar heads outside. The document classifies common types of surfactants and discusses their uses.