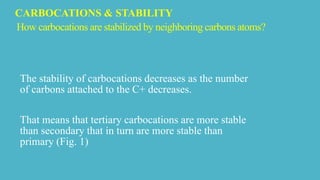
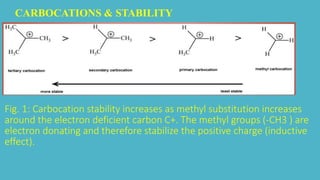
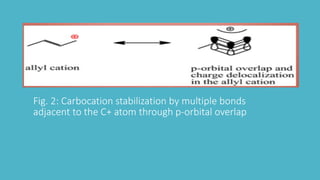
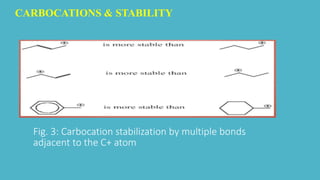
Carbocations are electron deficient carbon species with a positive charge that are very reactive and unstable. They can be stabilized by neighboring carbon atoms through an inductive effect, neighboring carbon-carbon multiple bonds through resonance effects, and neighboring atoms with lone pairs also through resonance effects. Tertiary carbocations are the most stable as methyl groups are good electron donors, while carbocations adjacent to carbon-carbon multiple bonds or atoms with lone pairs are also stabilized through charge delocalization via resonance.