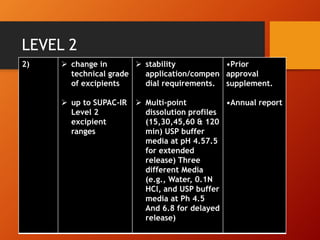
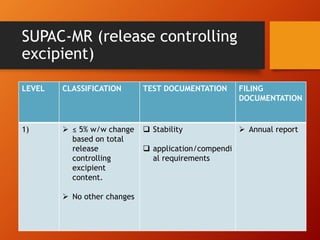
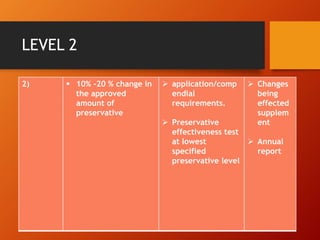
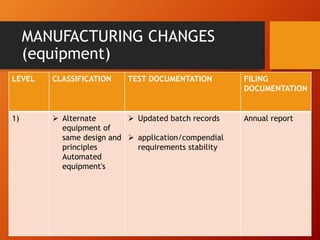
The document discusses Supplemental Abbreviated Changes to an Approved Application (SUPAC) guidelines for post-approval changes to pharmaceutical drug products. It defines three levels of changes - minor, moderate, and major - and provides recommendations for documentation and necessary filings based on the level of change for components/composition, manufacturing equipment, processes, batch size, and site changes. Minor changes may only require annual reporting, while major changes involving new excipients, processes, or sites would necessitate prior approval supplements and additional testing.