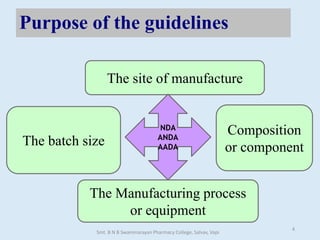
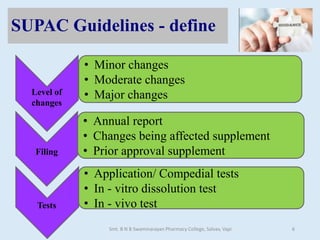
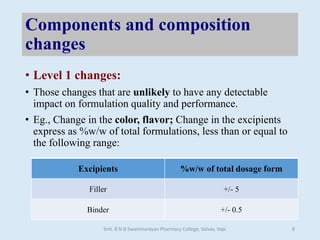
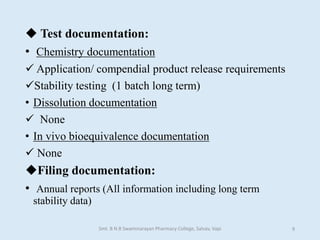
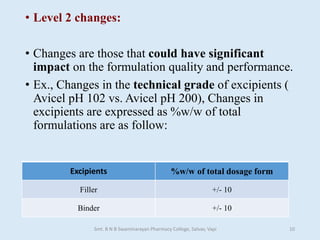
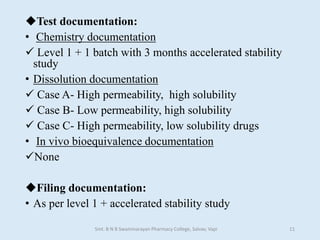
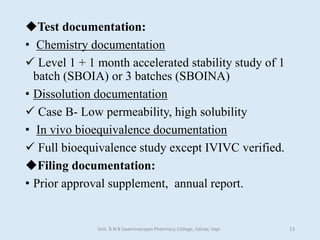
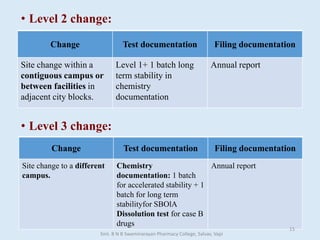
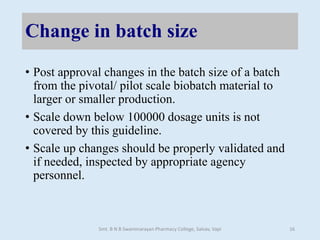
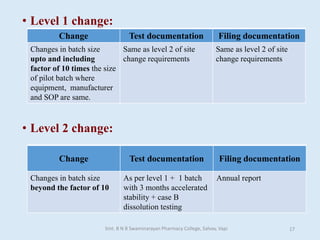
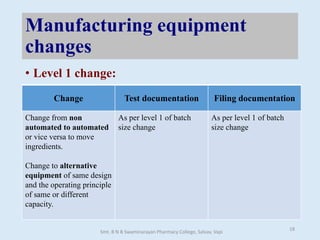
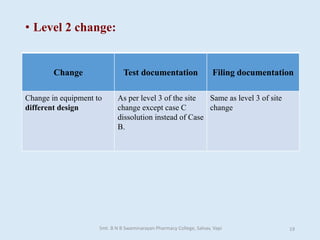
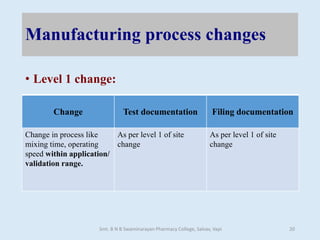
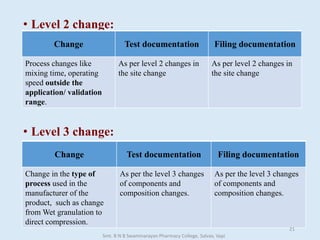
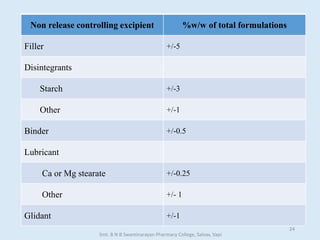
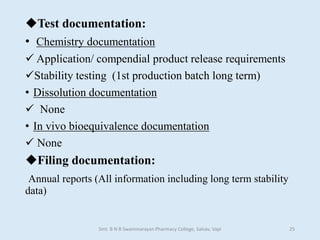
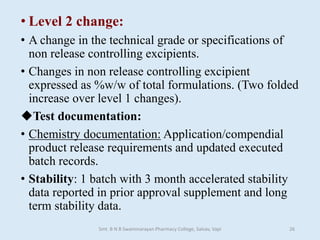
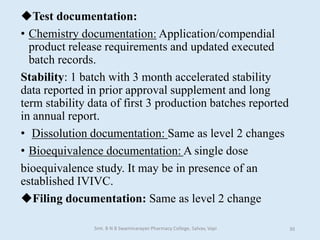
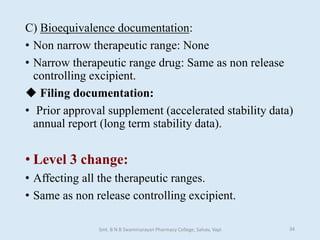
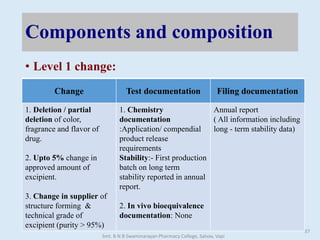
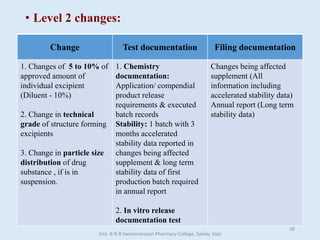
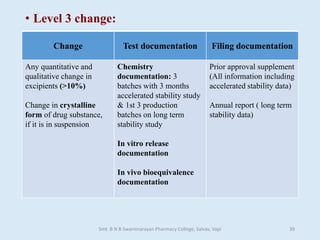
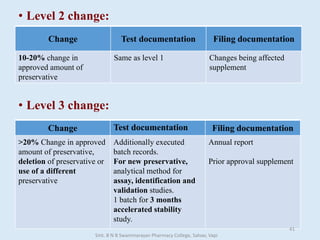
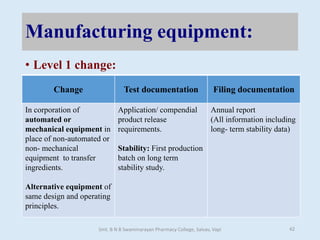
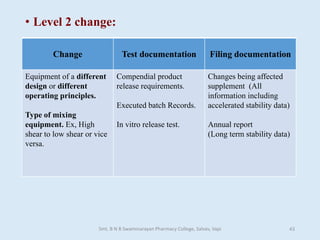
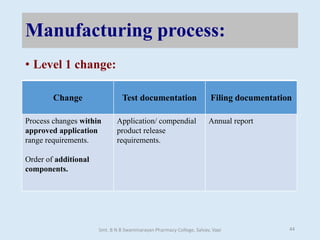
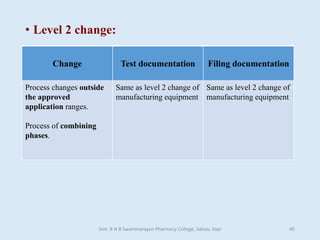
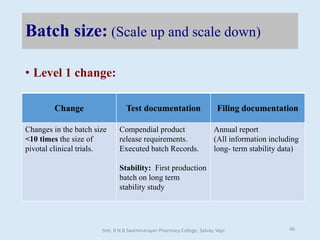
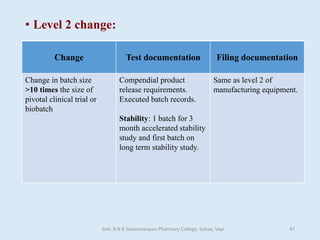
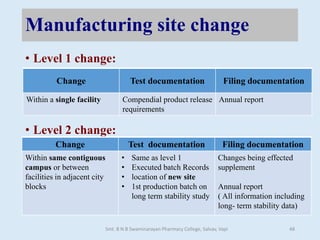
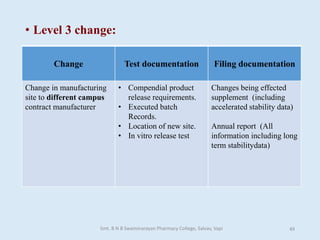
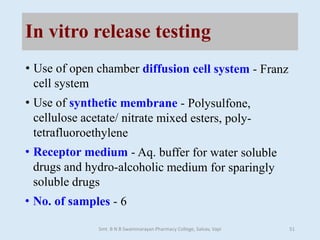
The document outlines the guidelines for Scale-Up and Post Approval Changes (SUPAC) in pharmaceutical manufacturing, detailing the process of increasing batch size and making approved changes in composition, equipment, and manufacturing sites. It categorizes changes into levels (1, 2, and 3) based on their potential impact on drug quality and performance, along with the required documentation for each level. The guidelines aim to facilitate the safe and efficient transition of pharmaceutical products through various changes while ensuring that regulatory standards are met.