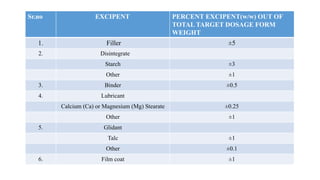
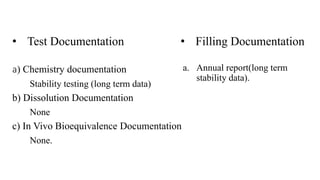
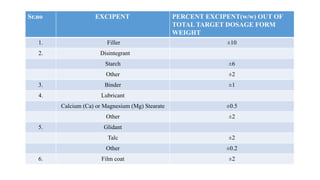
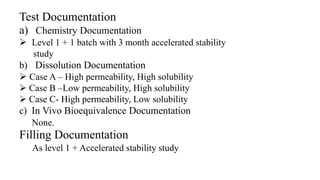
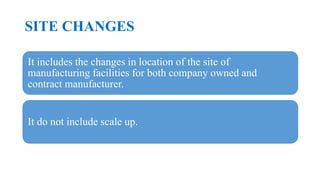
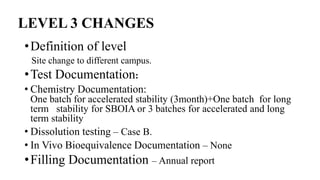
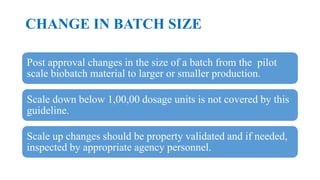
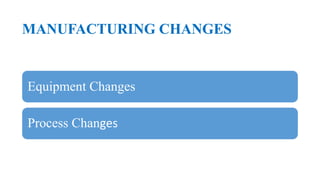
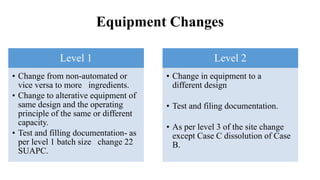
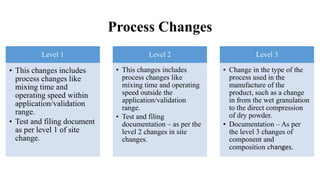
This document summarizes guidance on scale up and post-approval changes for pharmaceutical products. It outlines 3 levels for various changes based on their potential impact. Level 1 changes are unlikely to impact quality or performance. Level 2 changes may significantly impact quality or performance. Level 3 changes require full bioequivalence testing. The guidance covers changes to components, manufacturing site, batch size, equipment, and processes. Documentation requirements increase based on the level of change, ranging from chemistry testing to full bioequivalence studies.