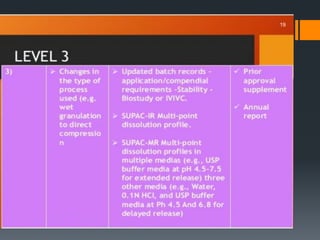
The document outlines the SUPAC (Scale Up and Post Approval Changes) guidelines established by the FDA for pharmaceutical firms to follow when making changes to the manufacturing process of approved drug products. It details different levels of changes (minor, moderate, major) and the corresponding testing and documentation required for site and batch size alterations. The guidelines aim to ensure compliance with good manufacturing practices while facilitating efficient updates to production processes.