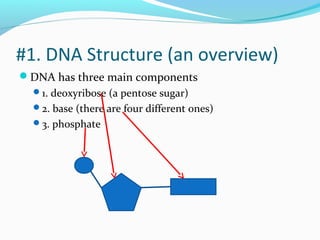
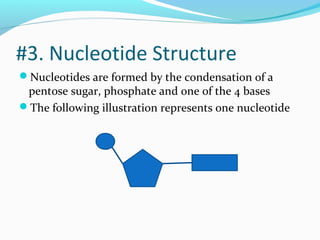
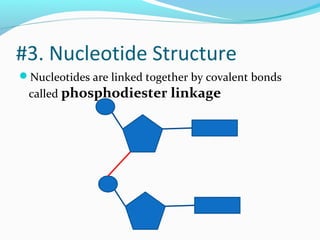
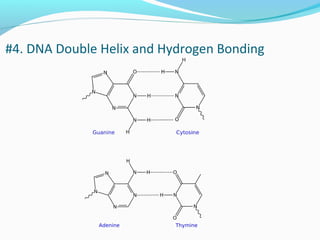
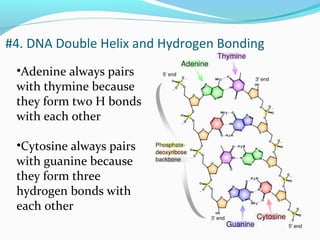
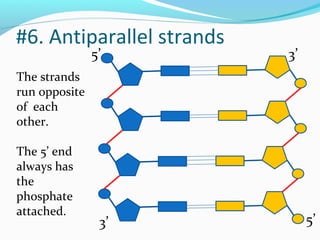
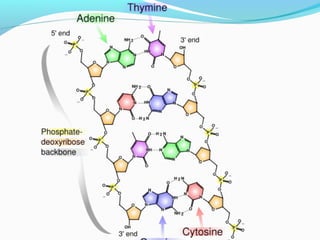
The document provides an overview of DNA structure. It discusses that DNA is composed of deoxyribose, phosphate groups, and four nitrogenous bases. The bases, adenine, guanine, cytosine, and thymine, form pairs through hydrogen bonding between complementary bases. Adenine pairs with thymine, and cytosine pairs with guanine. Nucleotides are formed from a pentose sugar, phosphate group, and one of the four bases. DNA takes the form of a double helix with the backbones made of alternating sugars and phosphates, and the bases forming rungs between the strands via hydrogen bonding of complementary base pairs. The two strands of DNA run in opposite directions, with the 5' end having