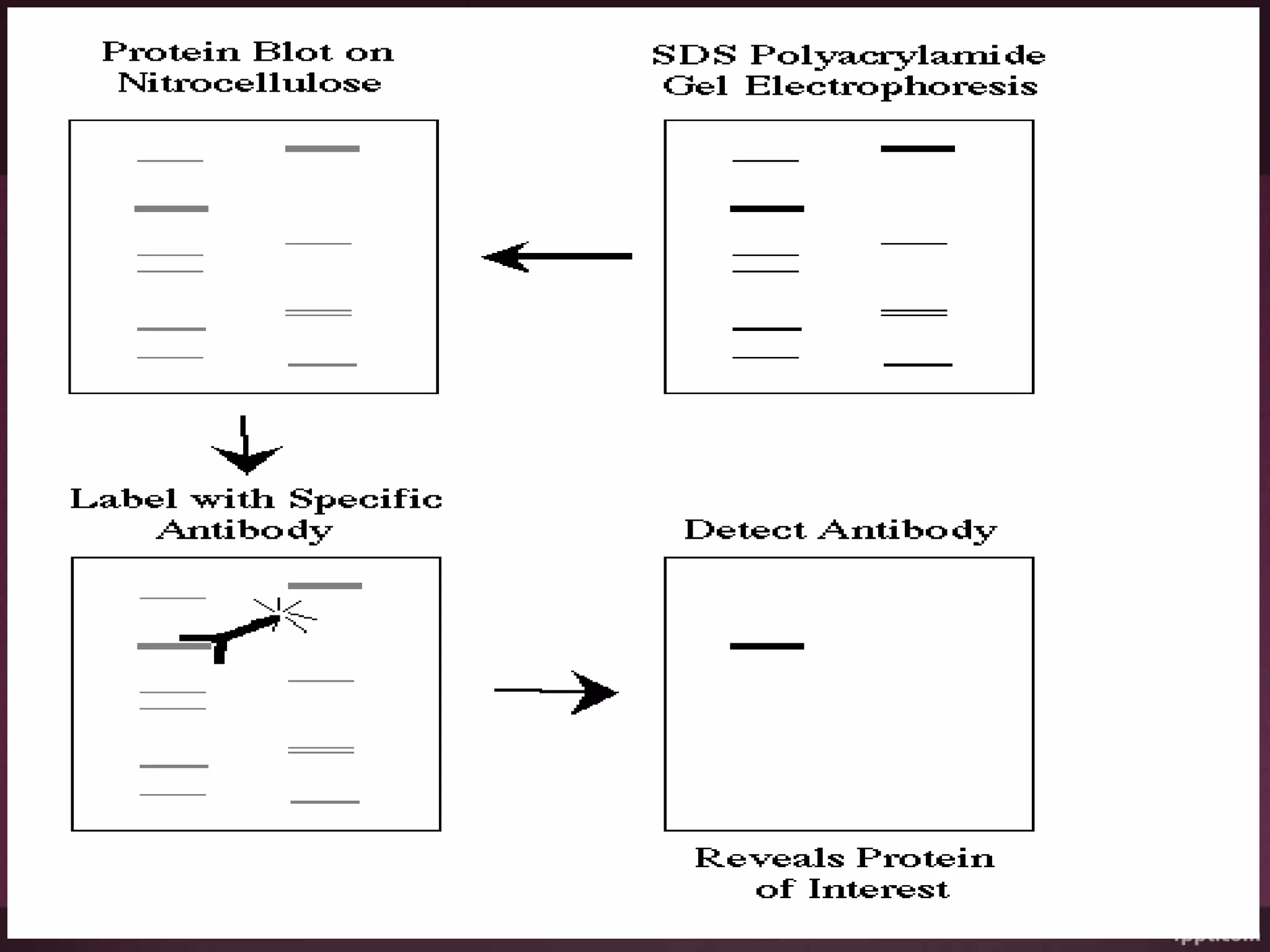
Blotting techniques such as Southern blotting, Northern blotting, and Western blotting allow for the transfer and detection of DNA, RNA, and proteins, respectively. Southern blotting involves separating DNA fragments via gel electrophoresis, transferring them to a membrane, and using a probe for hybridization to detect specific DNA sequences. Northern blotting detects RNA transcripts by separating RNA on a gel, transferring it to a membrane, and labeling it with a probe. Western blotting identifies proteins by separating them on an SDS-PAGE gel, transferring them to a membrane, and using primary and secondary antibodies to detect specific proteins based on antigen-antibody binding. These techniques have various applications in research, diagnostics, and forensics