Embed presentation
Downloaded 179 times

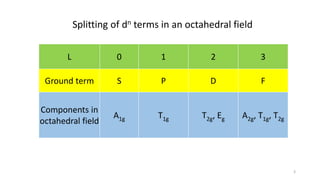
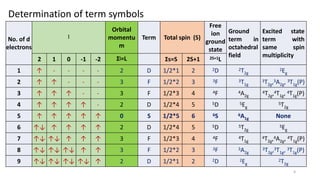

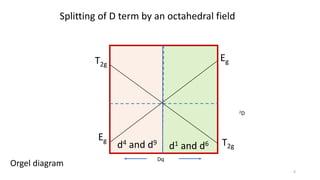
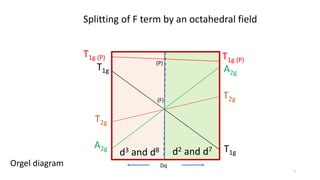
The document discusses spectroscopic term symbols, which represent the energy, angular momentum, and spin multiplicity of atoms, particularly in relation to hydrogen. It details how these symbols are defined for different atomic orbitals (s, p, d, f) and explains the splitting of d and f terms in an octahedral field. Additionally, it includes information on determining term symbols and presents Orgels diagrams for illustrating these concepts.






