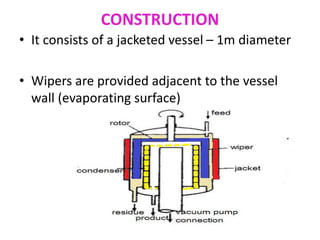
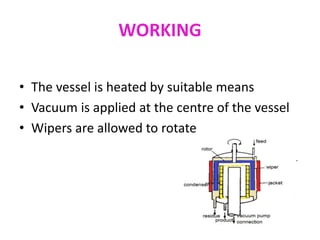
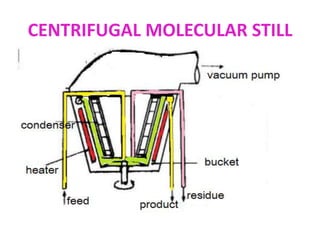
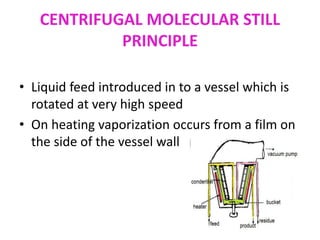
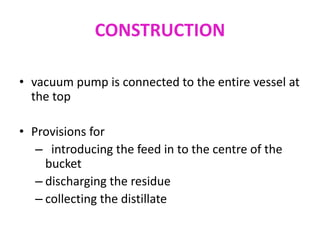
Molecular distillation is a distillation process that occurs under high vacuum conditions. This allows molecules to travel through the vapor phase without intermolecular collisions before condensing individually on a nearby surface. There are two main types of molecular stills - falling film stills and centrifugal stills. Falling film stills use a heated surface and wipers to create a thin liquid film for evaporation, while centrifugal stills rotate a bucket at high speeds to create an evaporating liquid film on the inner wall. Both utilize a short path between evaporation and condensation surfaces to minimize molecular collisions in the vapor phase. Molecular distillation is useful for separating compounds with similar boiling points.