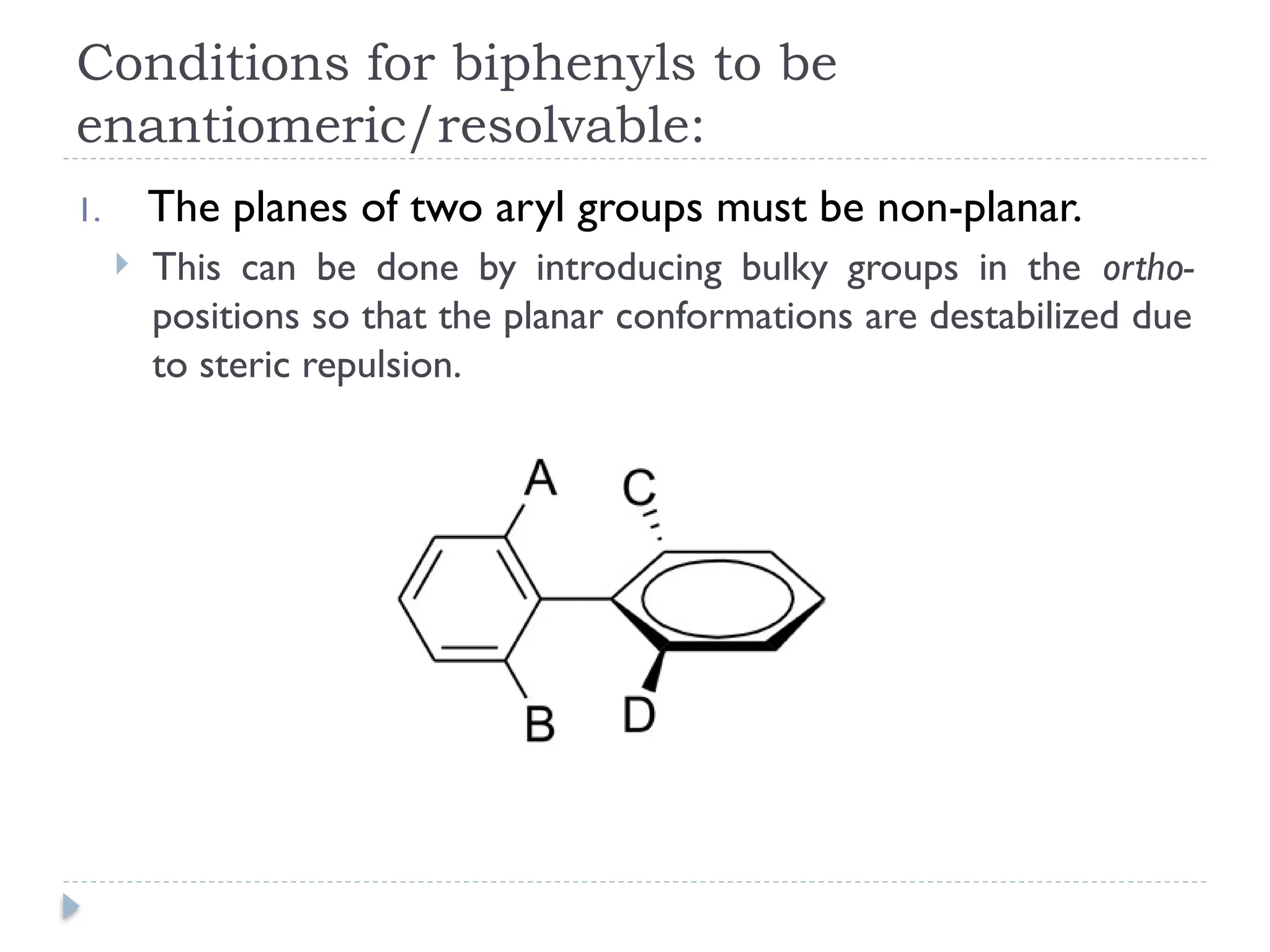
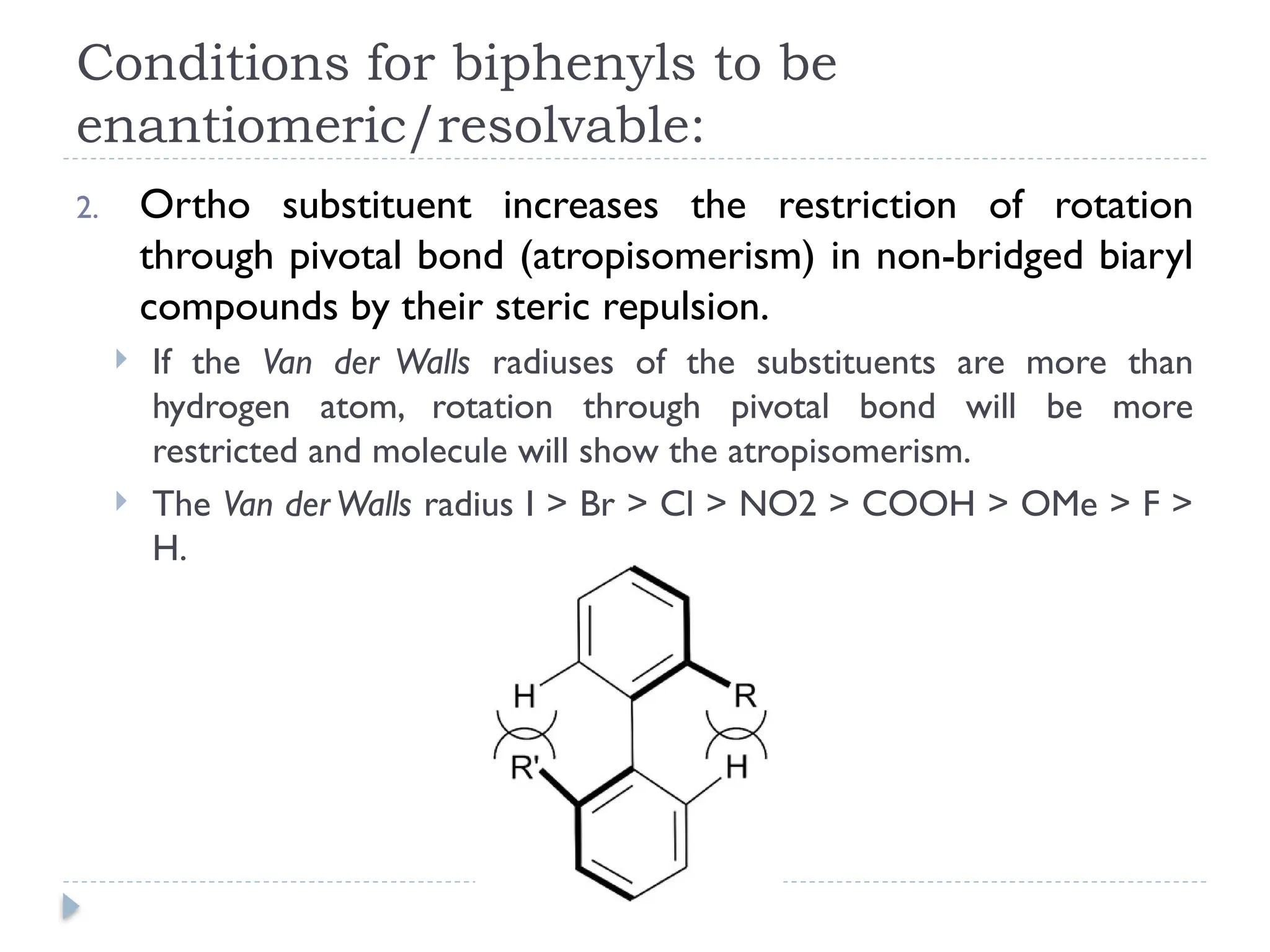
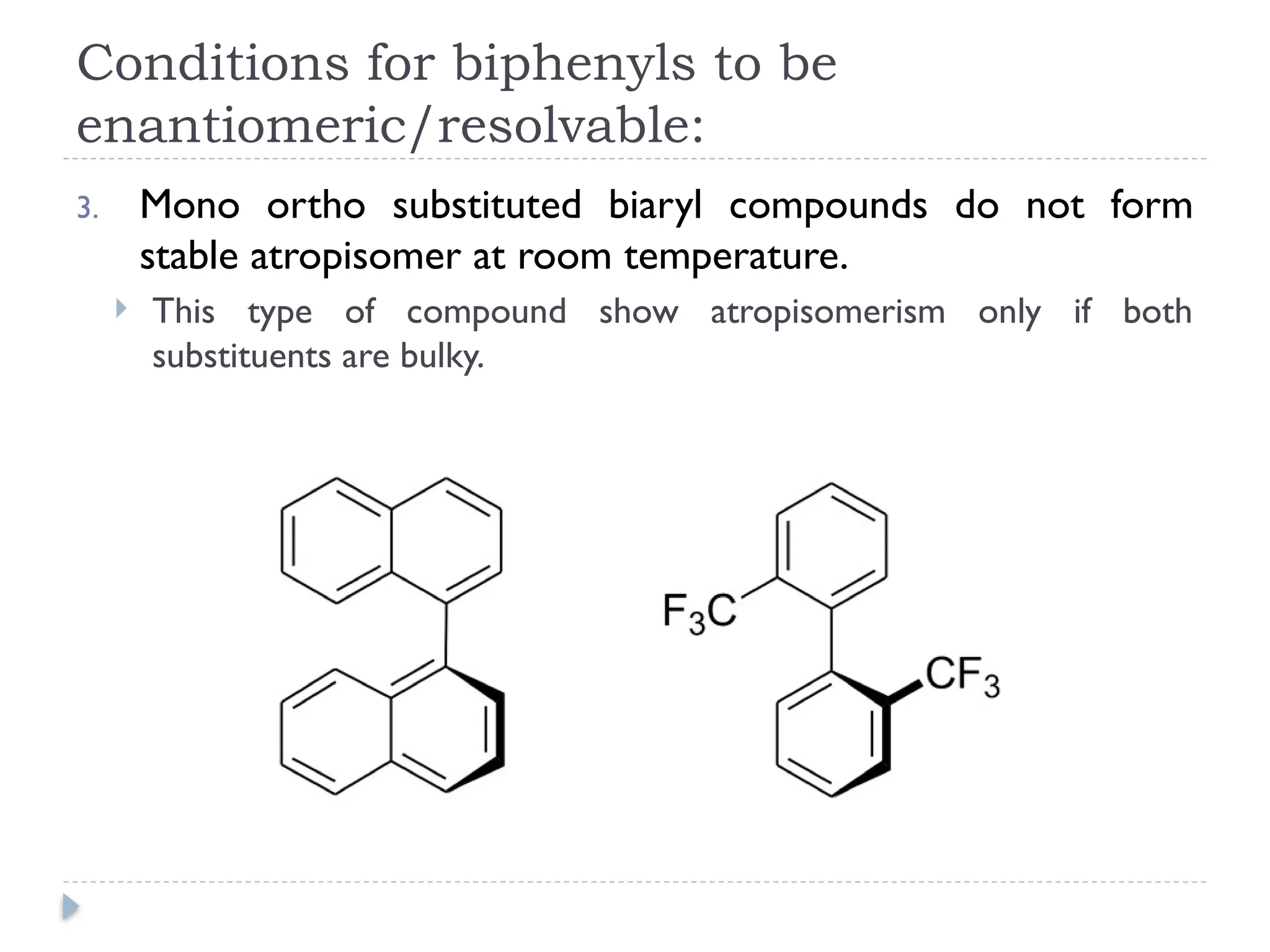
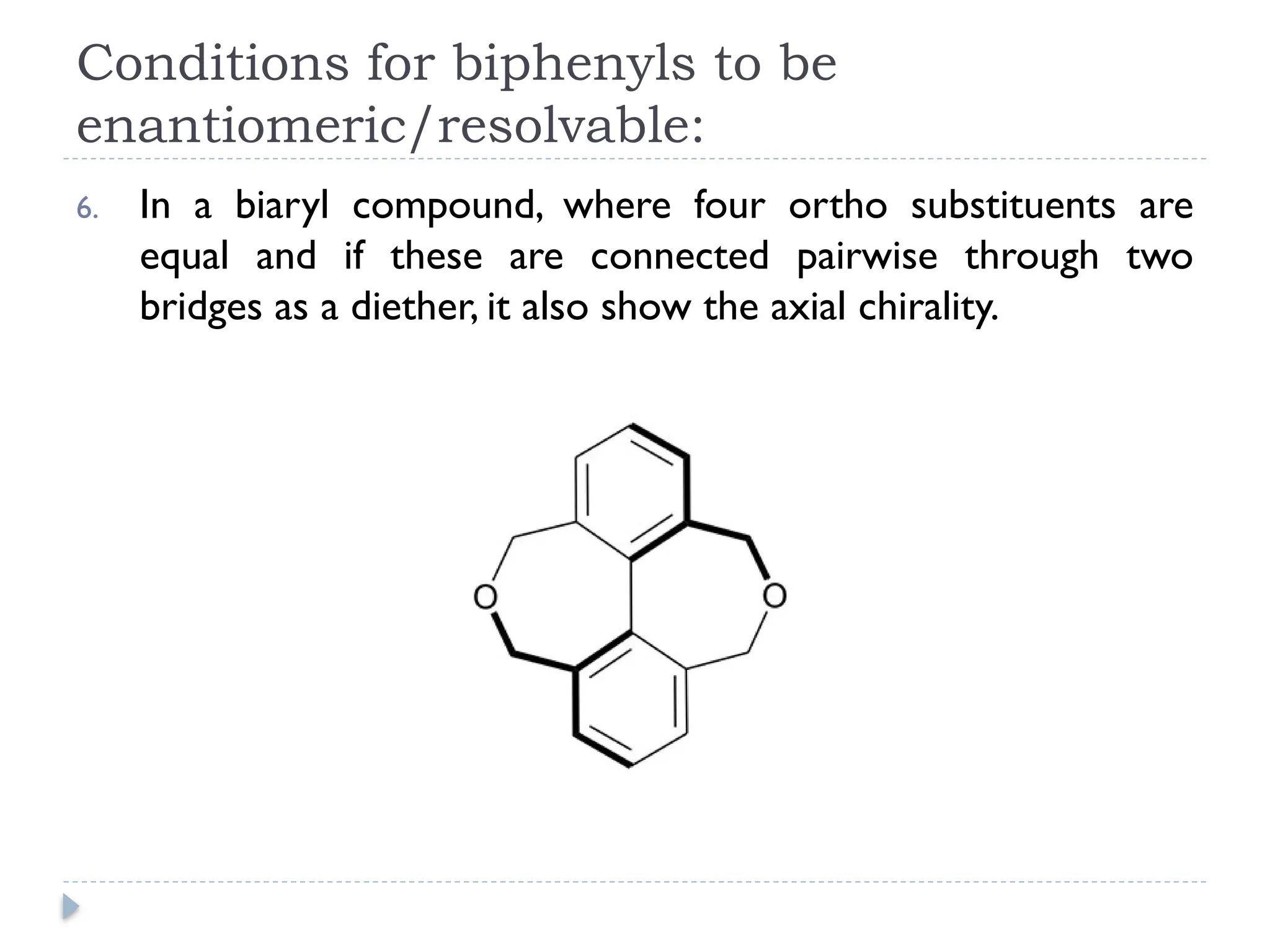
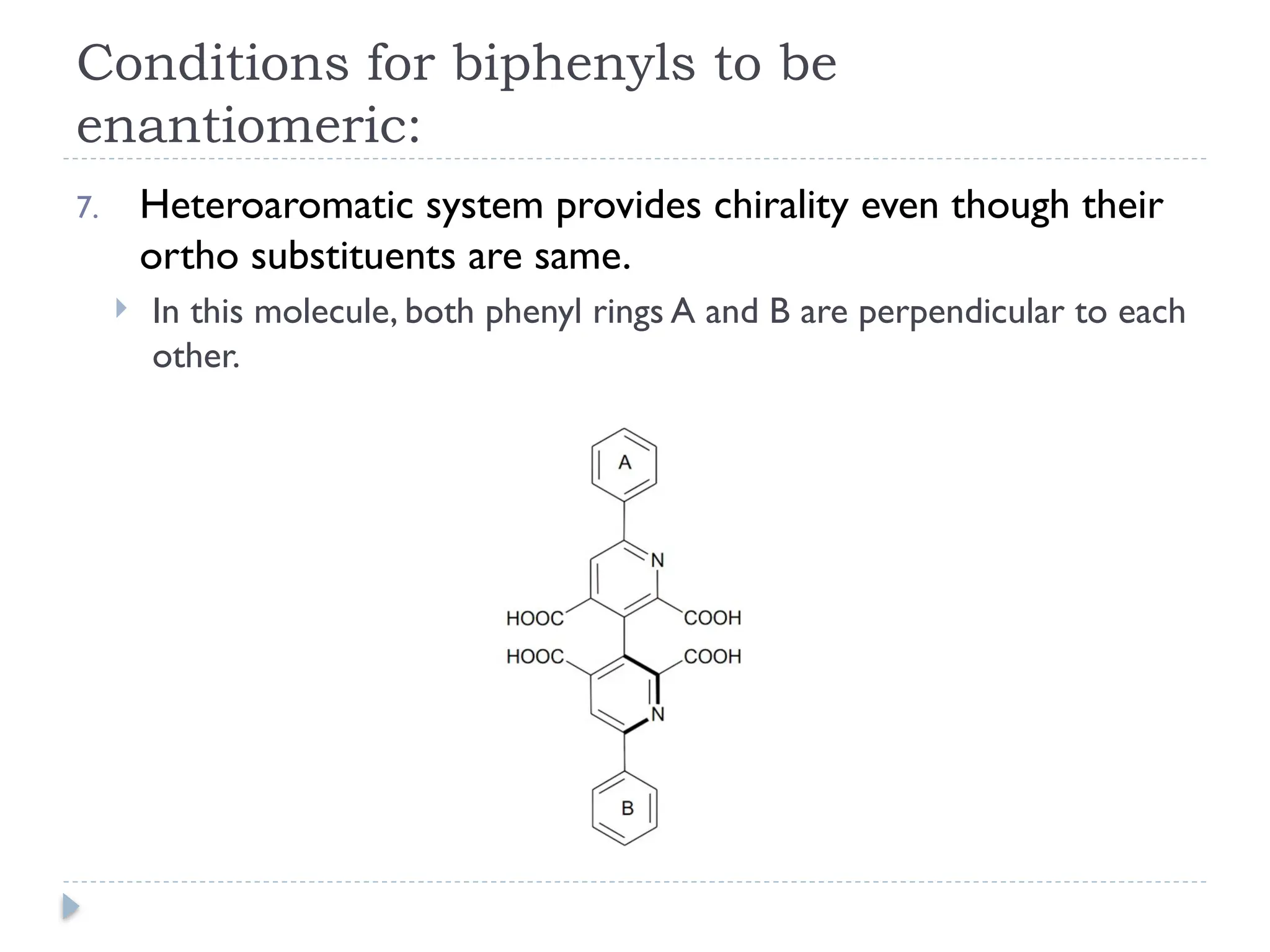
The document discusses atropisomerism in biphenyl compounds, emphasizing that biphenyl's chirality arises from ortho-position substitutions that restrict rotation around the bond connecting the two phenyl rings. It outlines various conditions necessary for biphenyls to be chiral and resolvable, including the need for bulky ortho substituents that create steric hindrance. Additionally, it examines the effects of substituent arrangement and size on the stability of atropisomers and racemization rates.