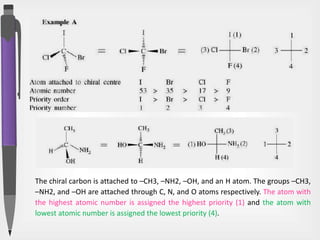
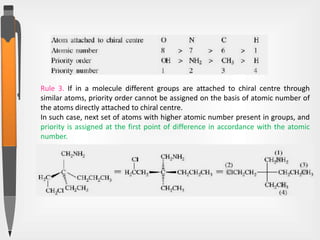
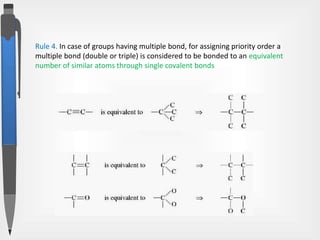
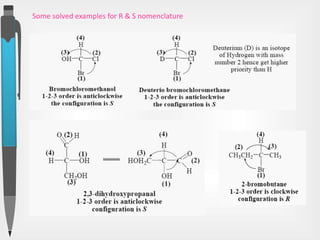
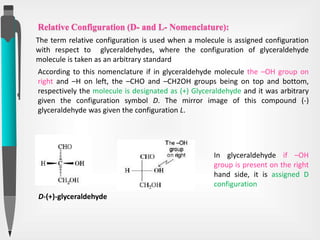
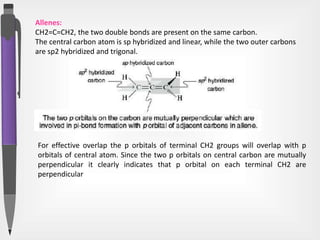
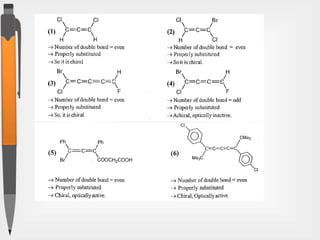
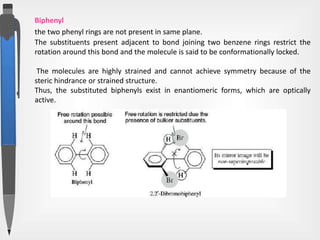
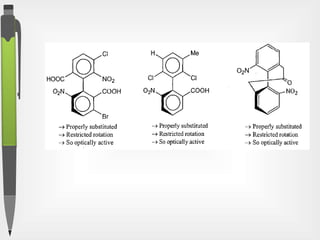
The document discusses the concepts of absolute and relative configuration in chiral molecules, focusing on the spatial arrangement of atoms around chiral centers. It explains the difference between the d/l (relative) and r/s (absolute) nomenclature systems for describing stereoisomers, including rules for assigning priorities to attached groups. Additionally, it addresses examples of chirality in molecules with or without stereogenic centers and introduces specific cases such as glyceraldehyde and the optical activity of allenes and biphenyls.