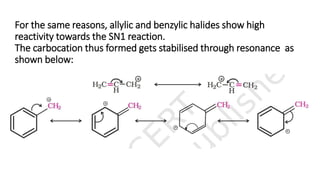
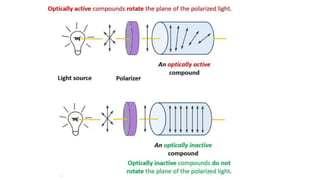
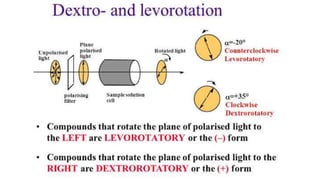
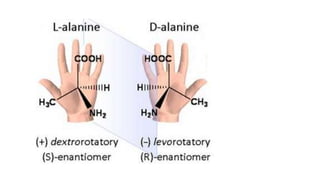
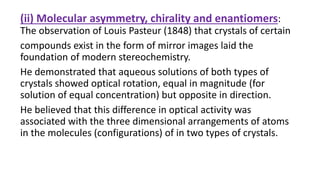
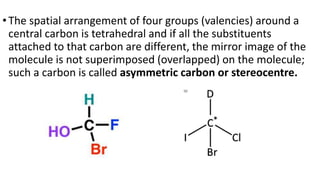
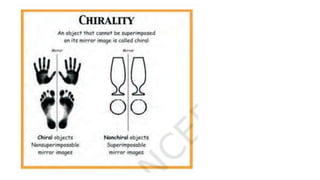
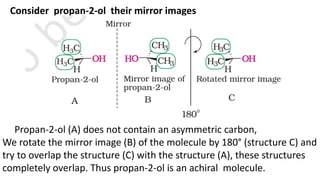
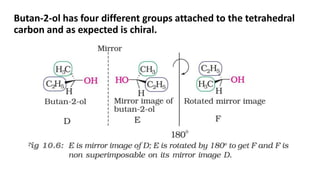
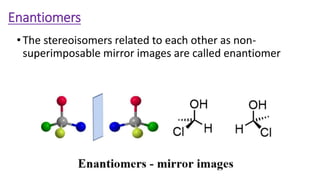
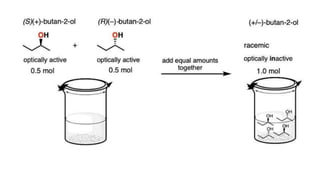
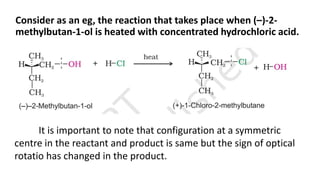
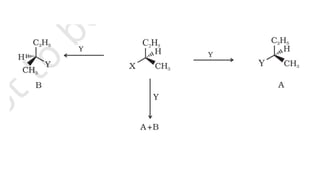
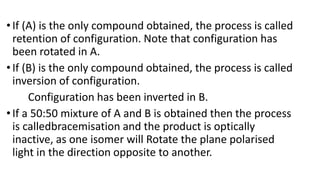
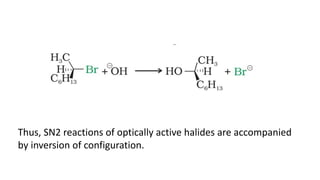
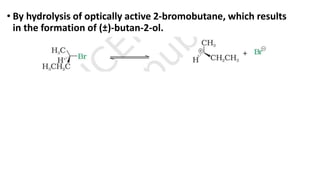
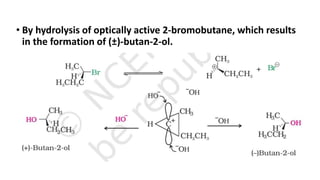
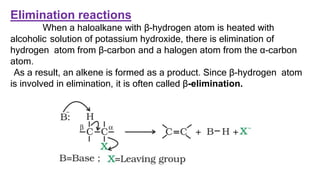
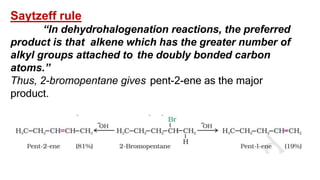
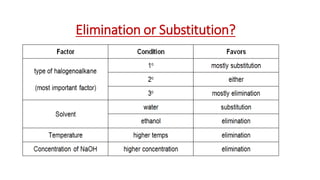
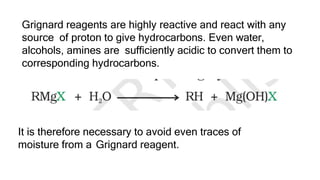
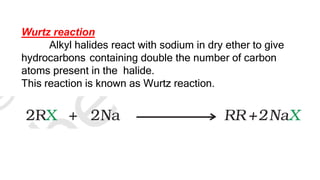
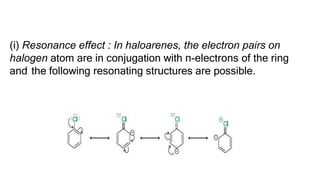
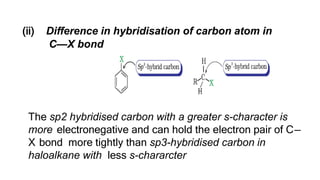
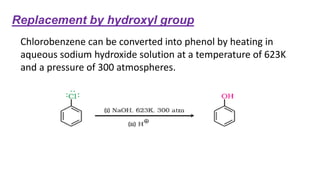
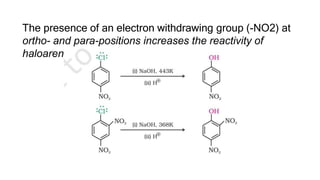
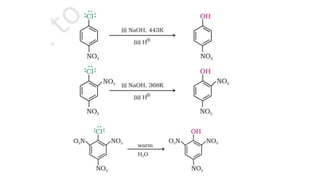
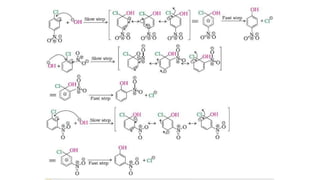
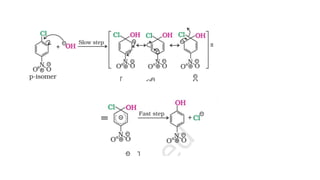
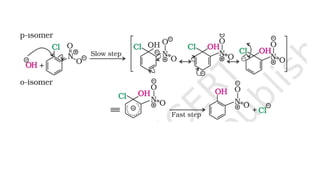
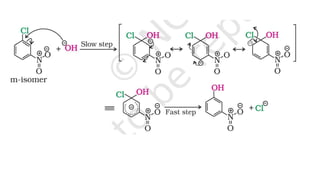
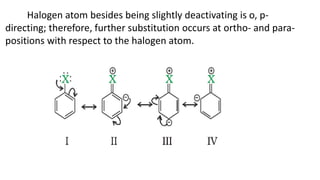
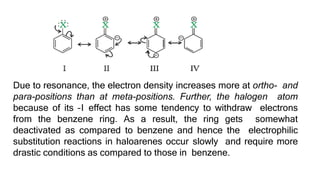
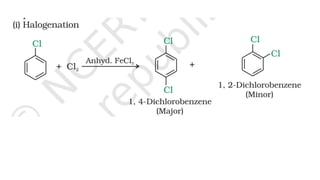
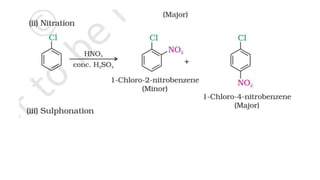
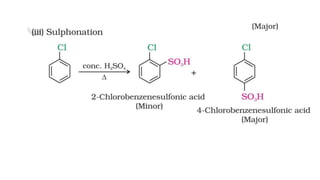
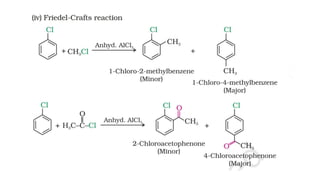
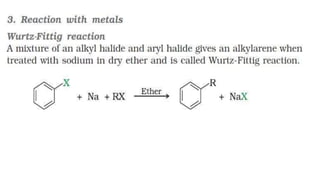
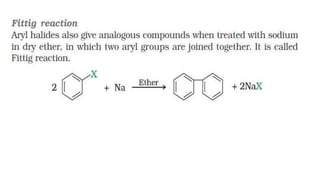
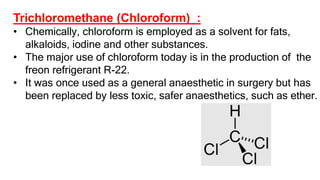
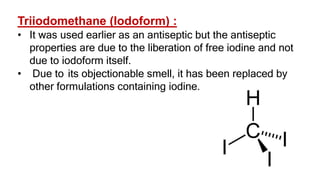
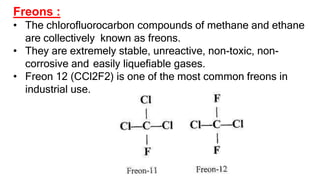
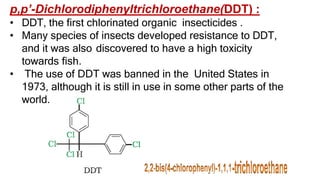
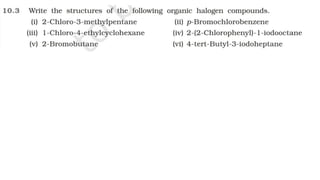
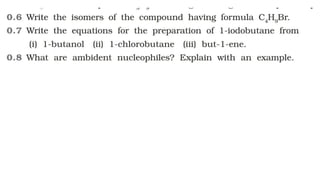
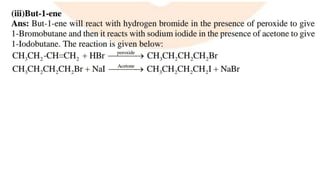
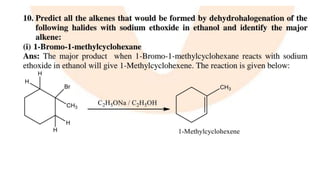
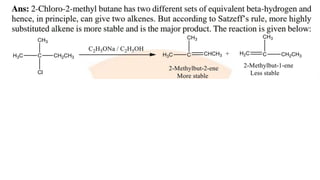
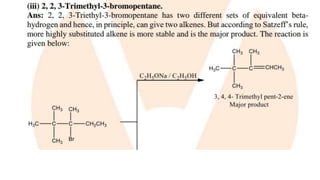
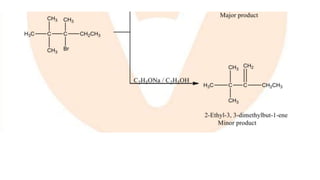
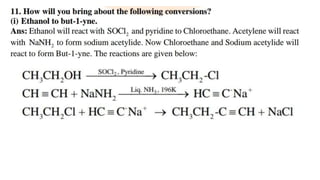
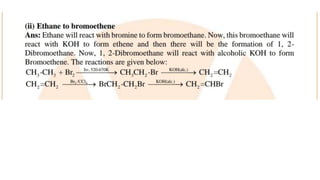
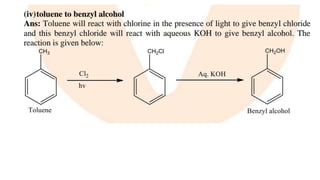
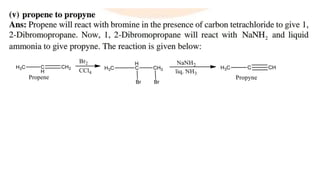
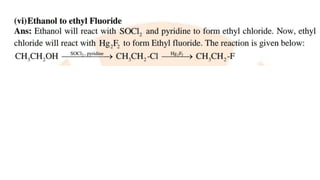
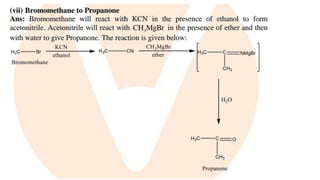
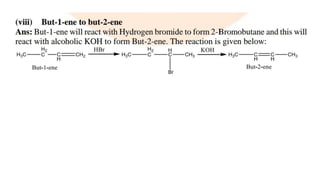
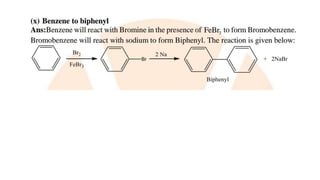
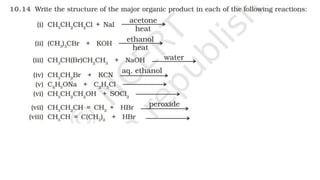
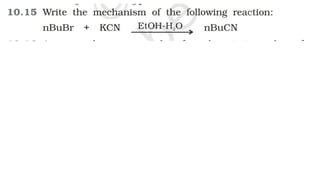
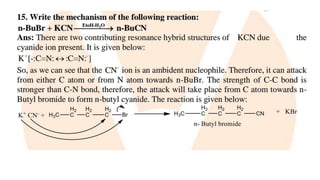
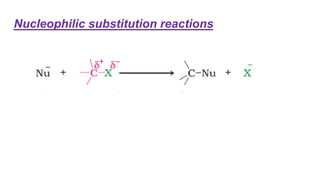
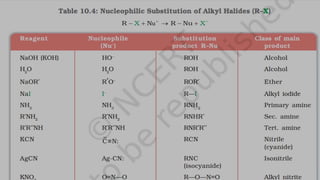
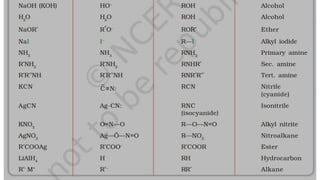
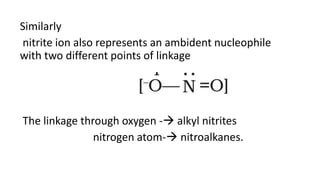
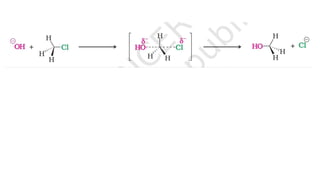
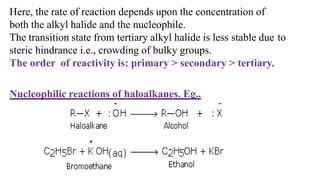
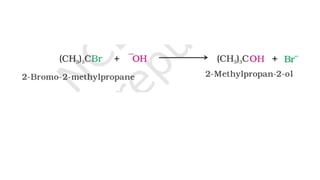
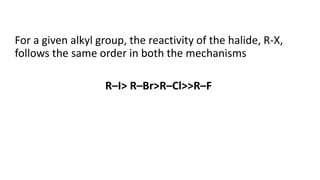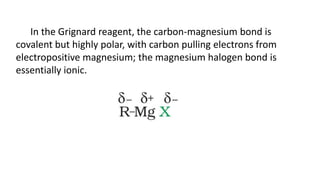The document summarizes the reactions of haloalkanes. It discusses three main categories of reactions: 1) Nucleophilic substitution, 2) Elimination reactions, and 3) Reactions with metals. It provides details on nucleophilic substitution reactions, including SN1 and SN2 mechanisms. It also describes elimination reactions, such as beta-elimination. Reactions with metals like Grignard reagents and Wurtz reactions are mentioned. Finally, it discusses reactions of haloarenes, including nucleophilic substitution and electrophilic substitution. Examples of polyhalogen compounds like dichloromethane and trichloromethane are also summarized.


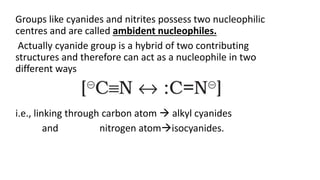

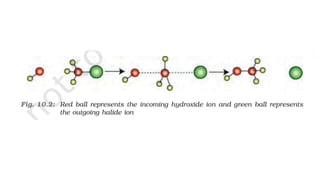




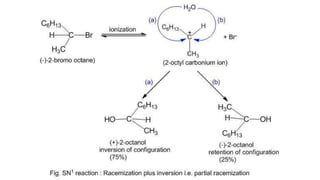
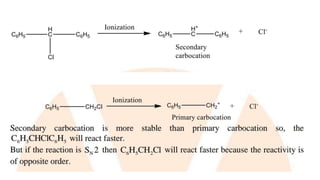
![• The first step is slow and is the rate-determining step. As the
nucleophile (Z-) is not involved in the rate-determining step, the
reaction depends only upon the concentration of alkyl halide
(RX) and is, therefore, a first order reaction.
Rate = k [RX]
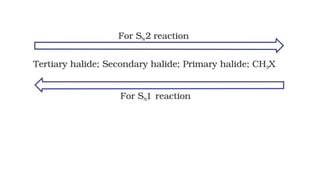
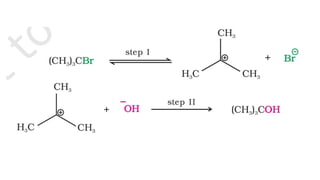
• The order of reactivity depends upon the stability of carbonium
ion formed in the first step. Since the 3° carbonium ion is most
stable, the ionization of tertiary alkyl halide is favored. The
order of reactivity for S1 reactionis,
tertiary > secondary > primary](https://image.slidesharecdn.com/presentation8-220820095452-3f15e14f/85/Presentation-8-pptx-19-320.jpg)