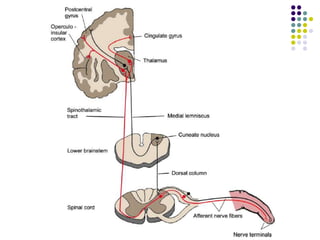
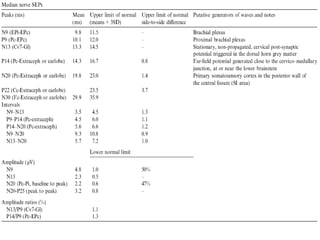
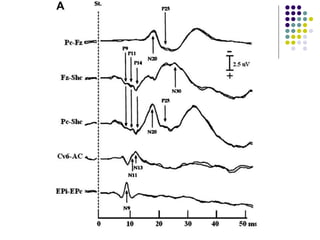
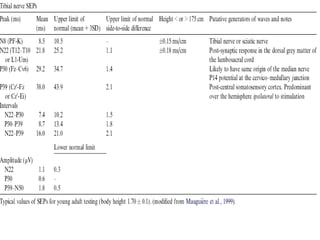
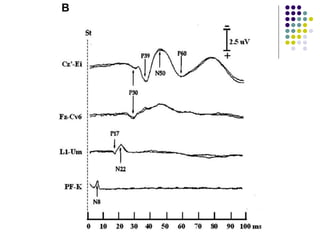
This document discusses somatosensory evoked potentials (SEPs), which are electrical signals generated in the nervous system in response to sensory stimuli. SEPs reflect the activation of neural structures along somatosensory pathways. They are recorded using electrodes on the scalp and spine in response to electrical stimulation of peripheral nerves. SEP waves are labeled according to their polarity and latency. Clinical uses of SEPs include evaluating peripheral nerves and central somatosensory pathways, localizing lesions, and monitoring patients in intensive care and during surgery. Abnormal SEPs can indicate disorders of the peripheral or central nervous system.