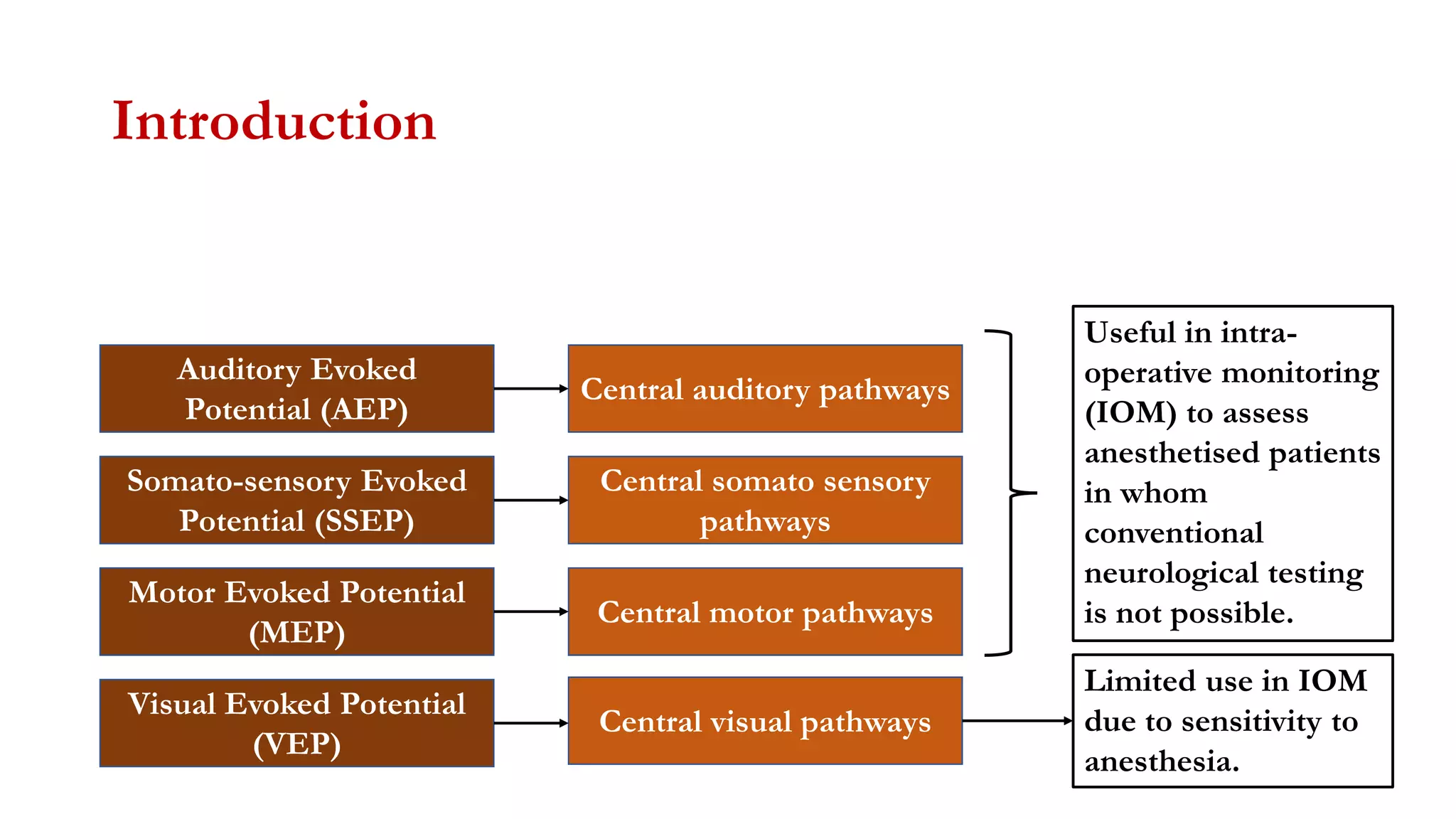
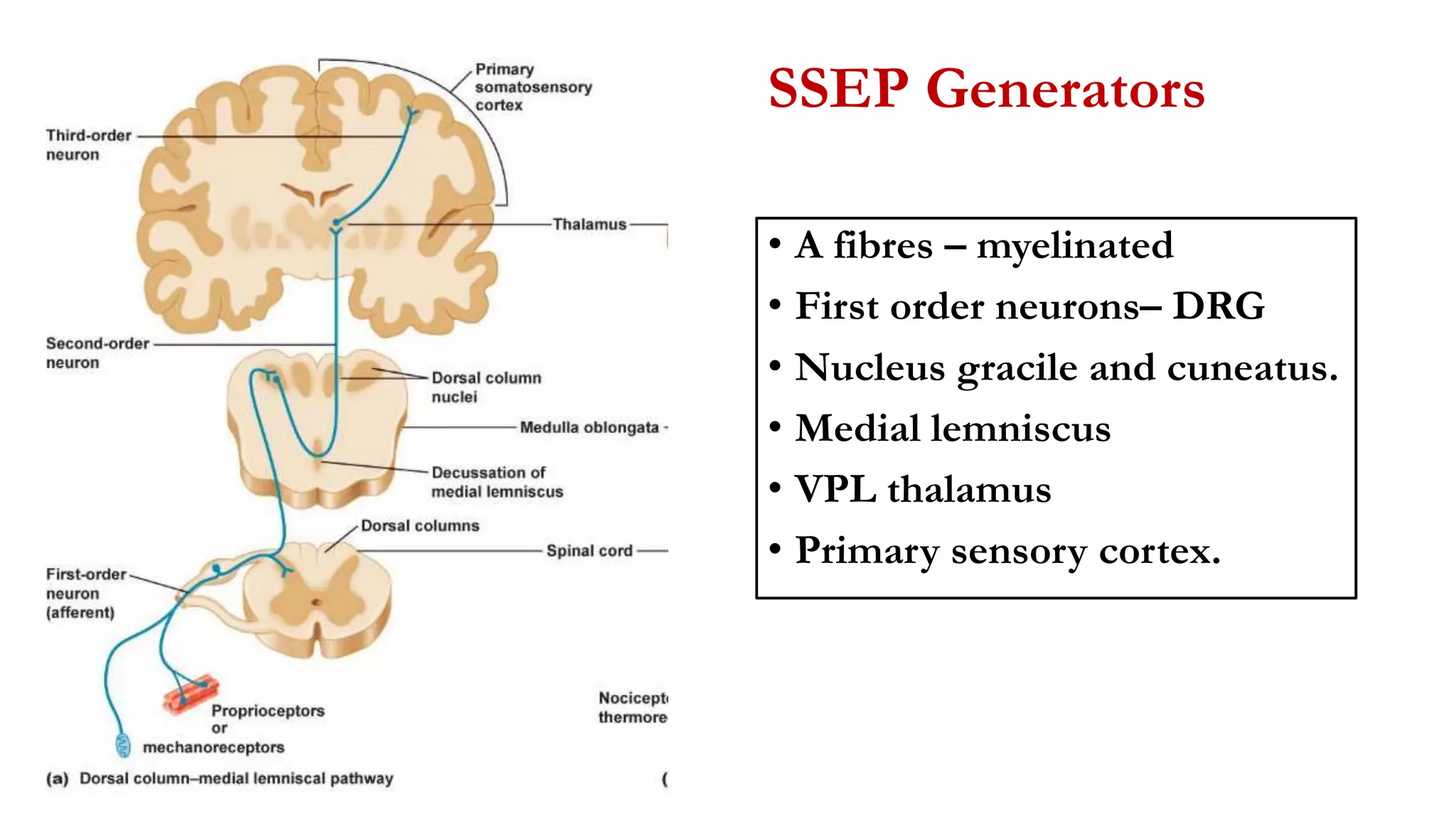
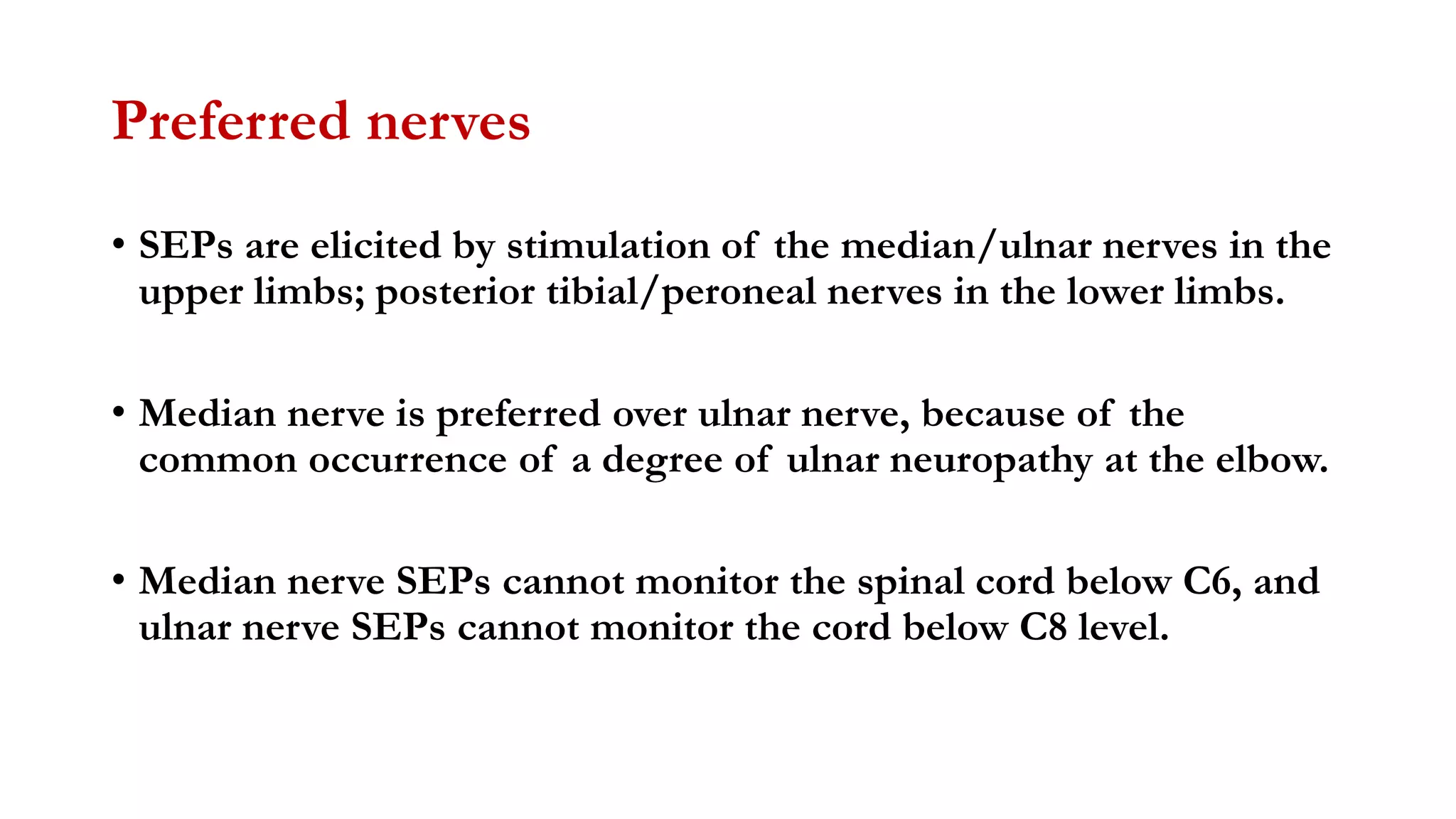
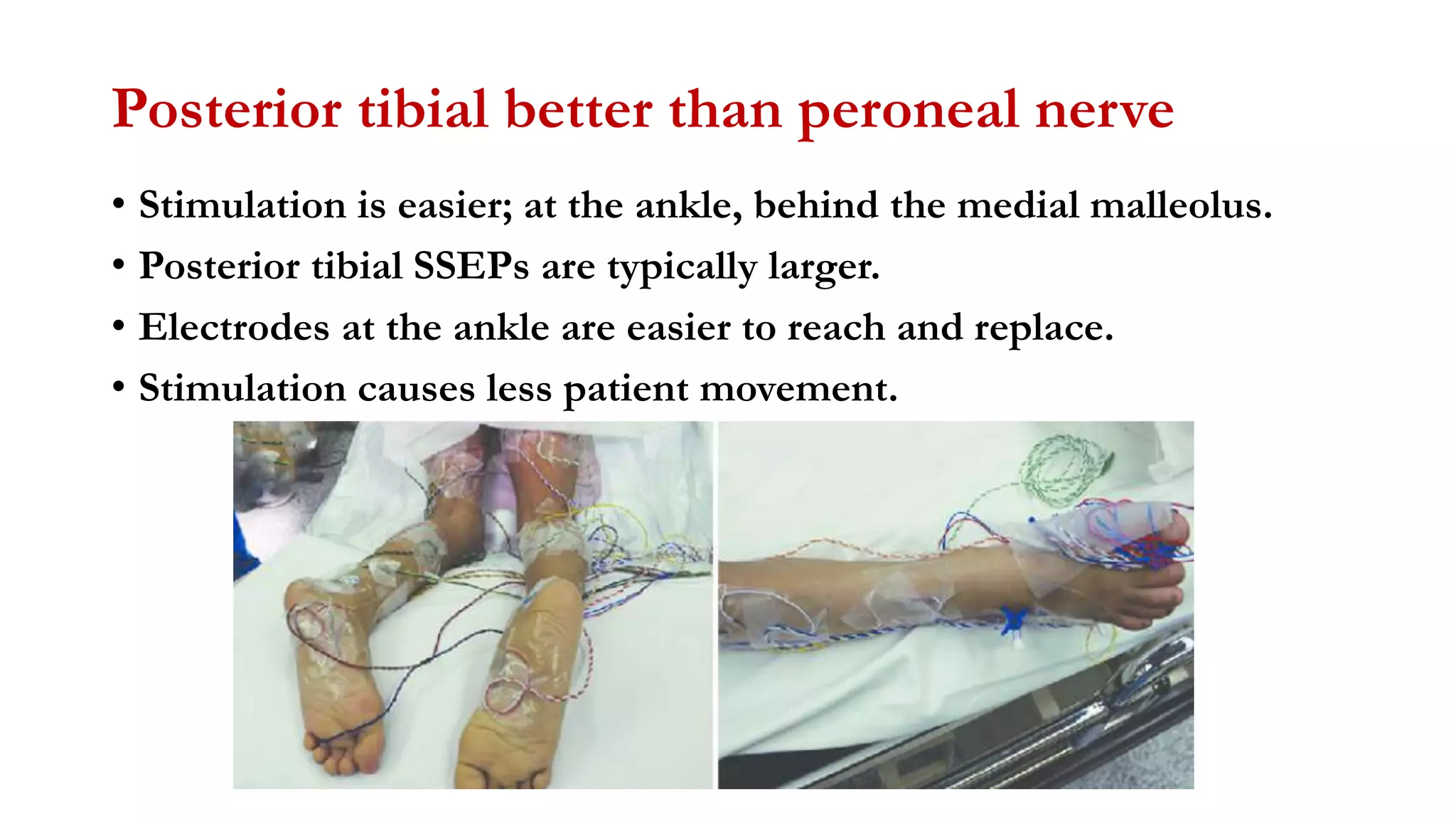
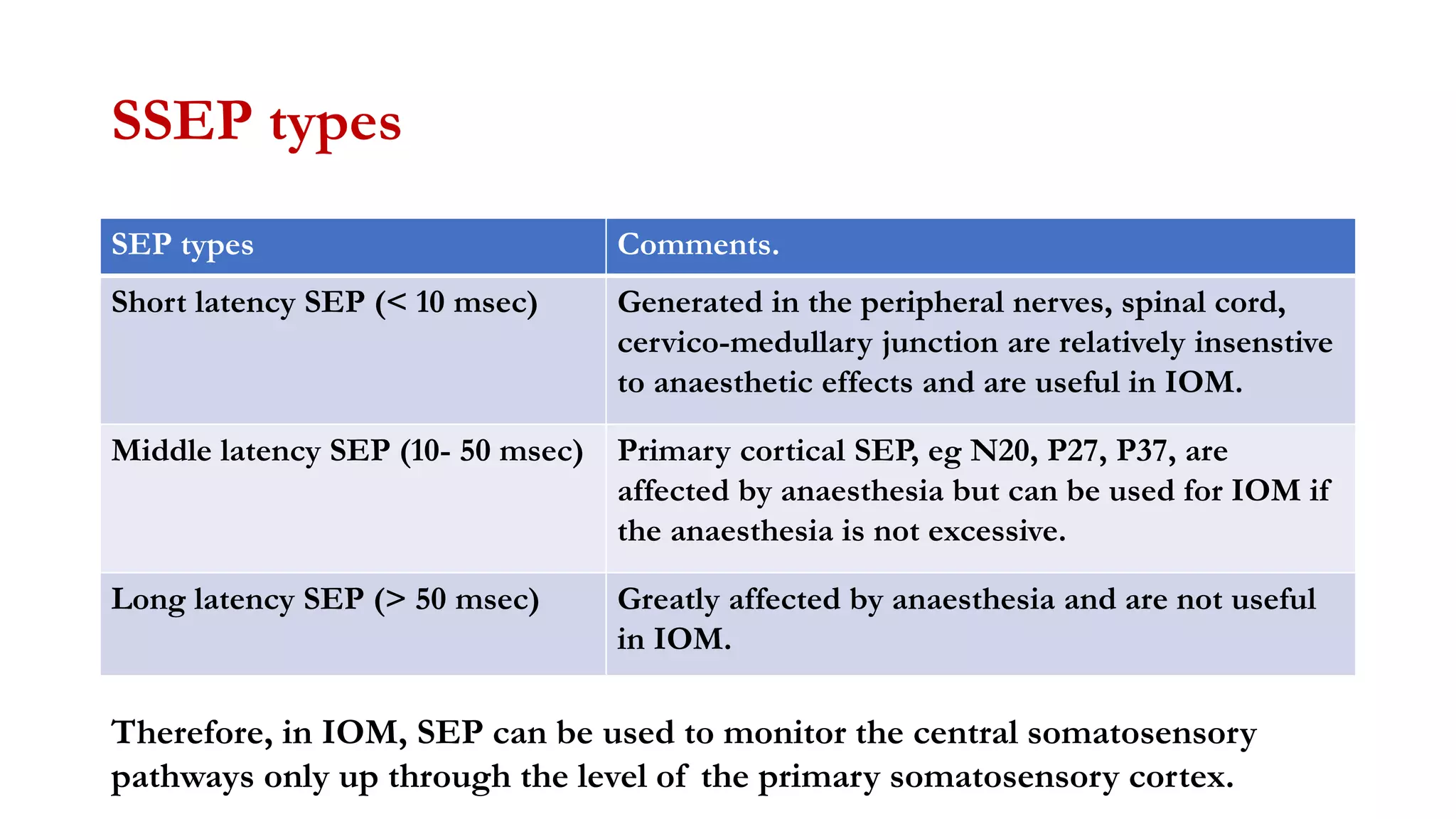
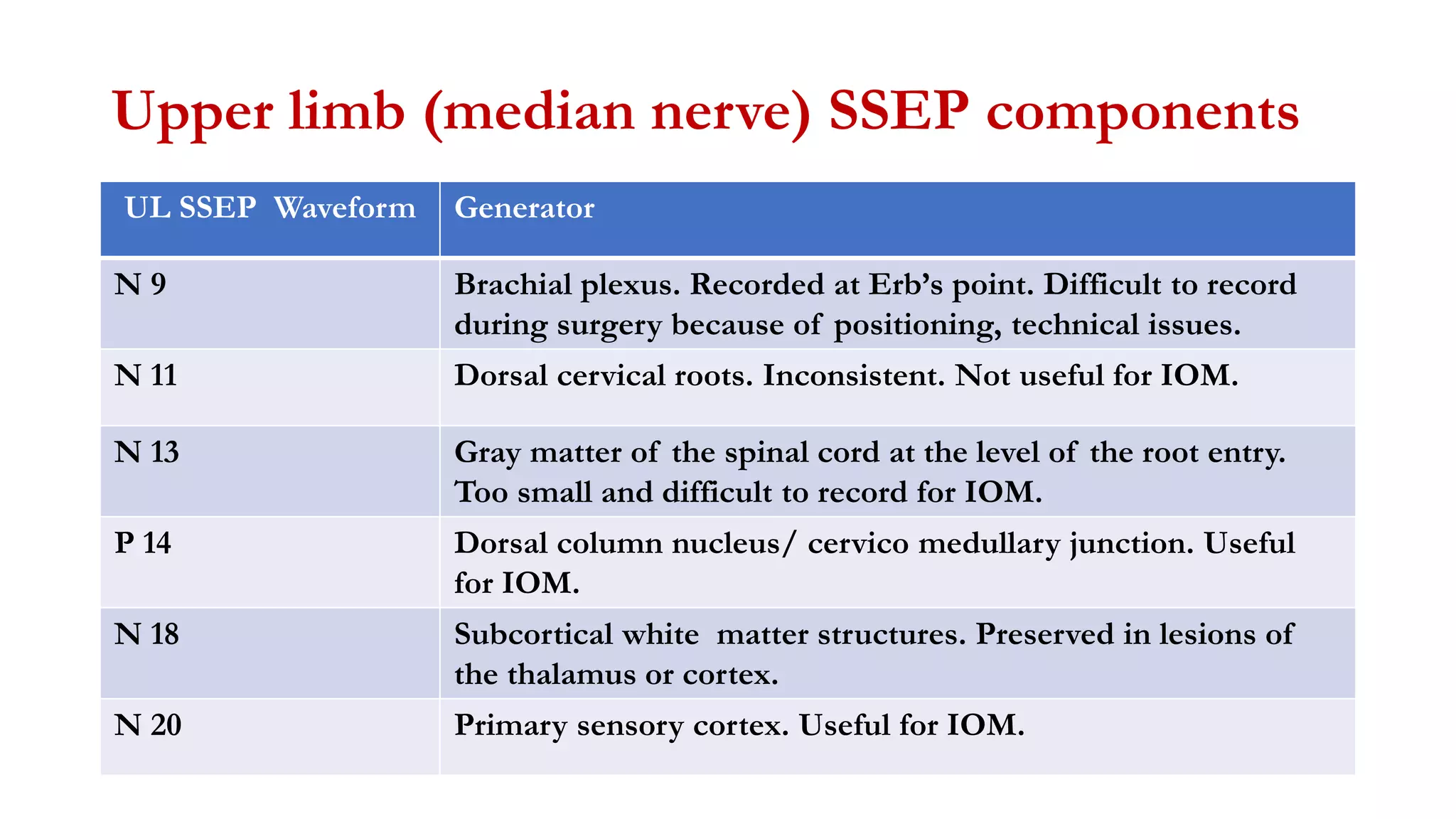
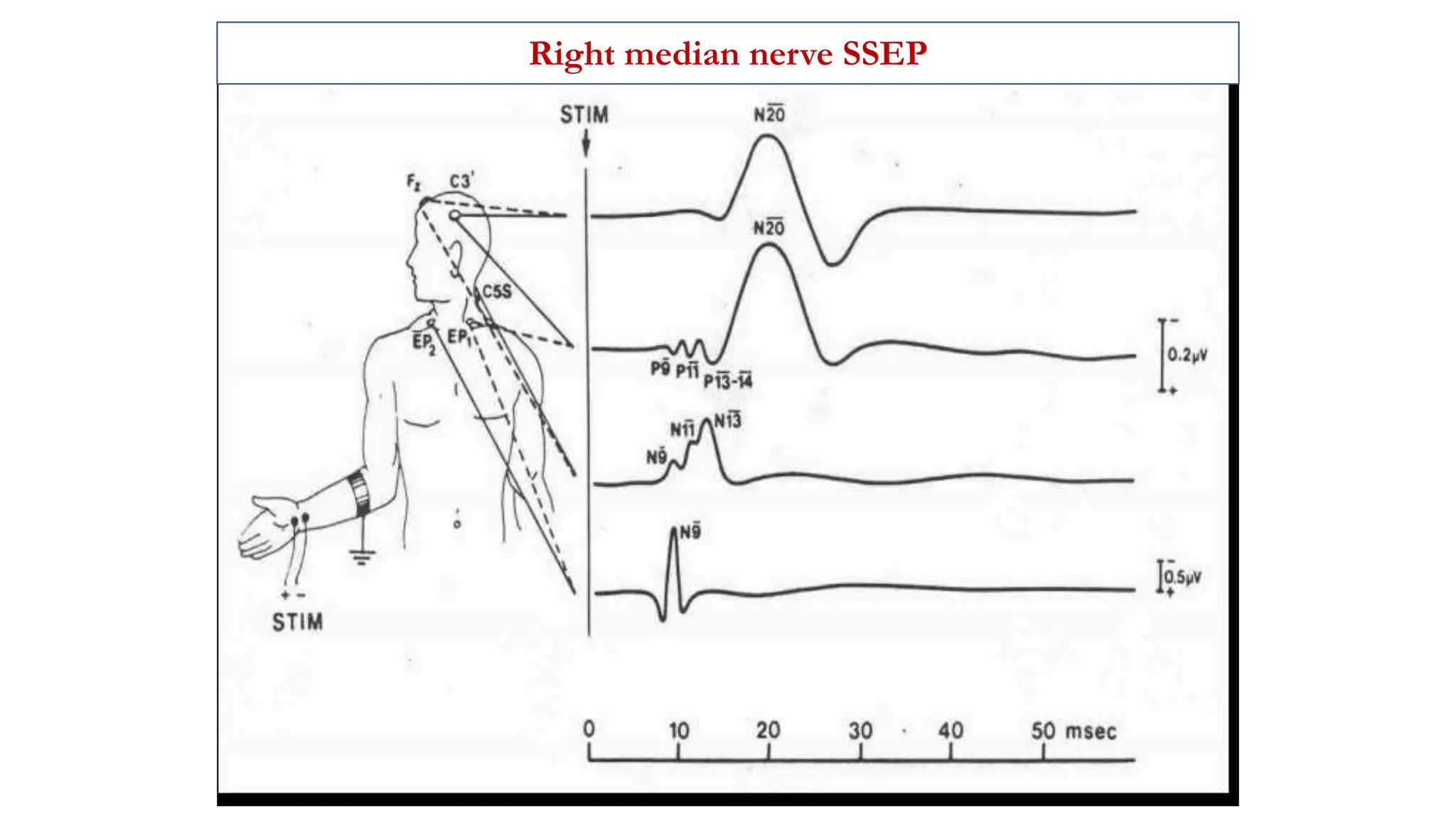
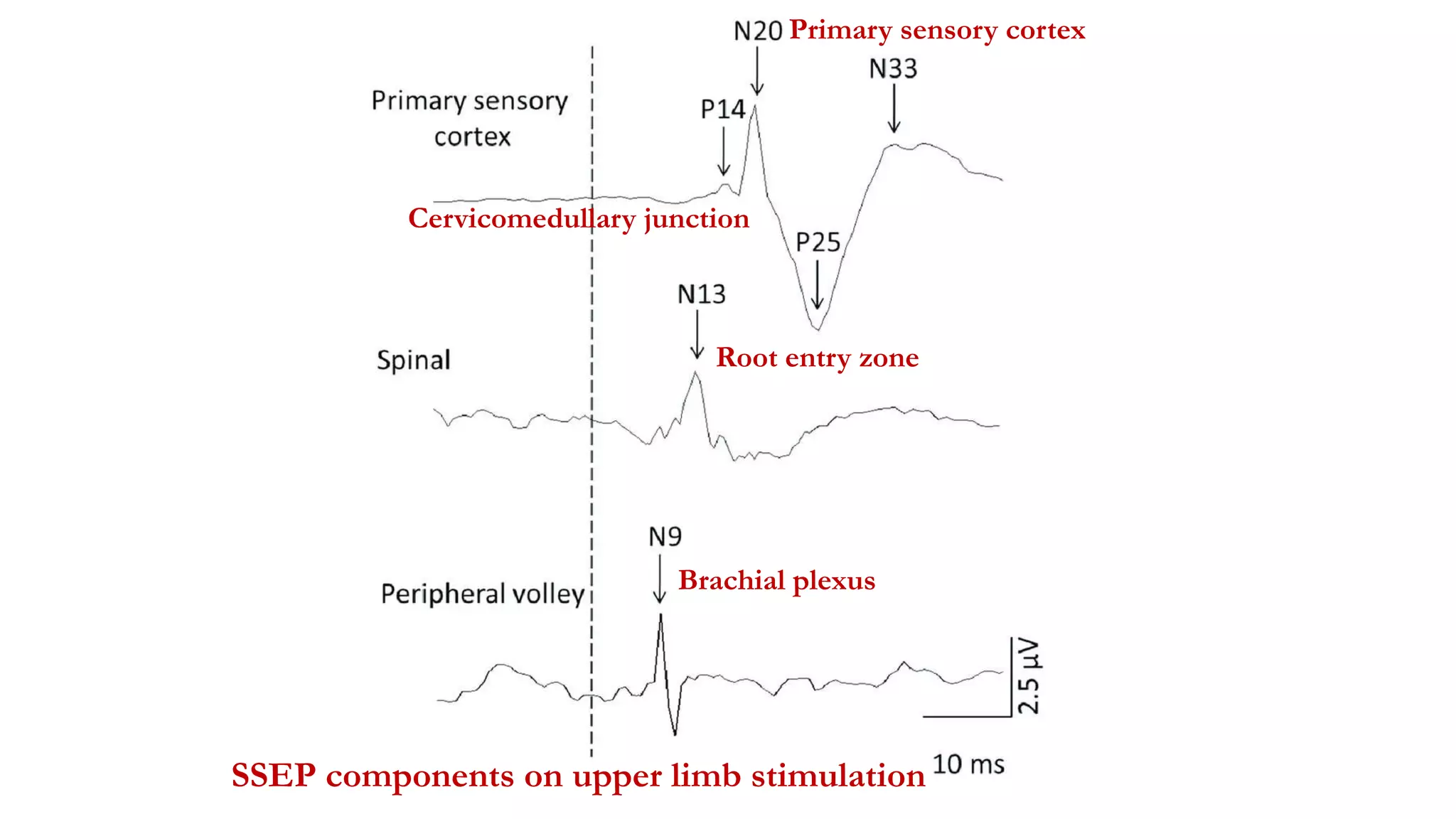
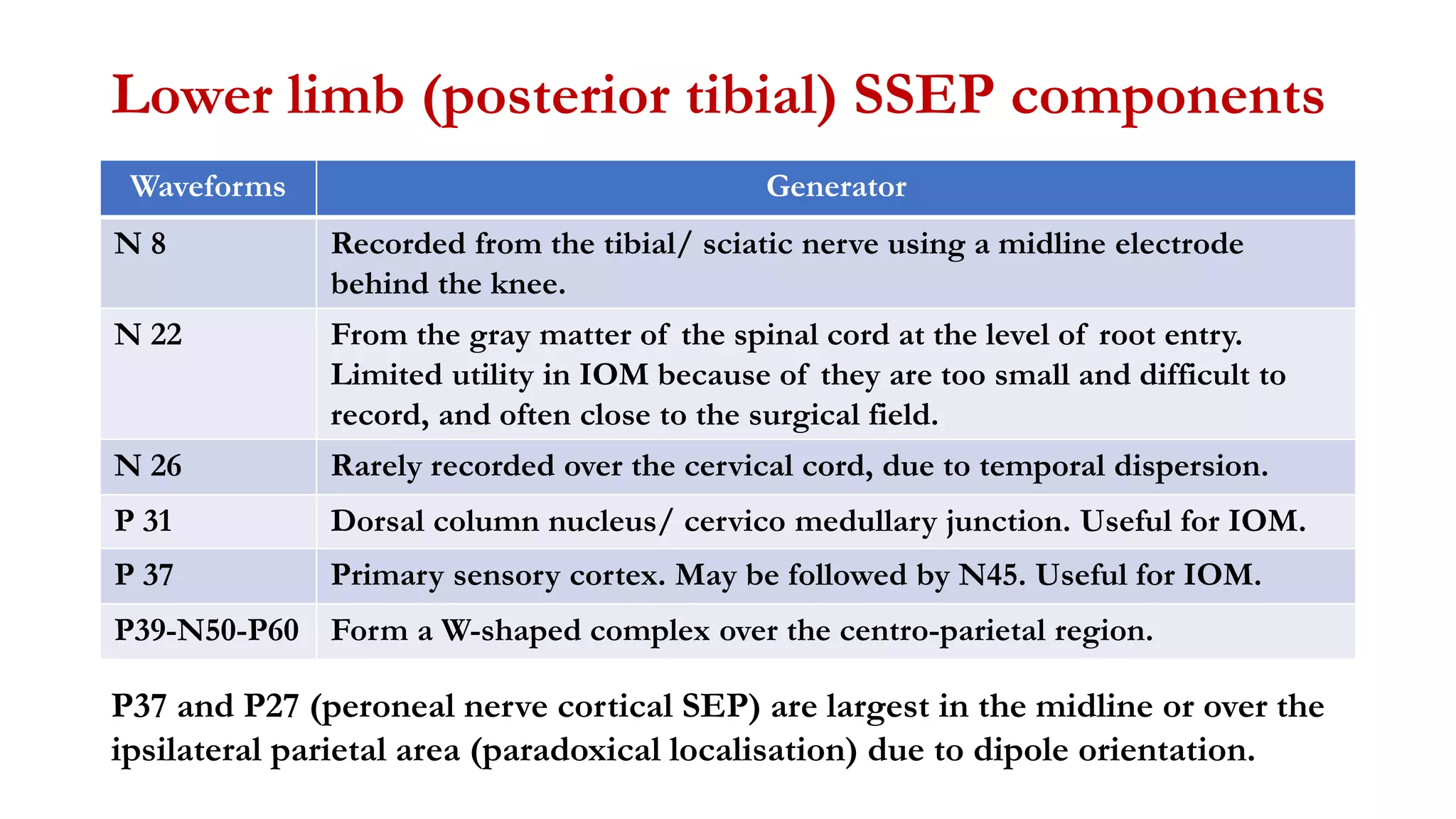
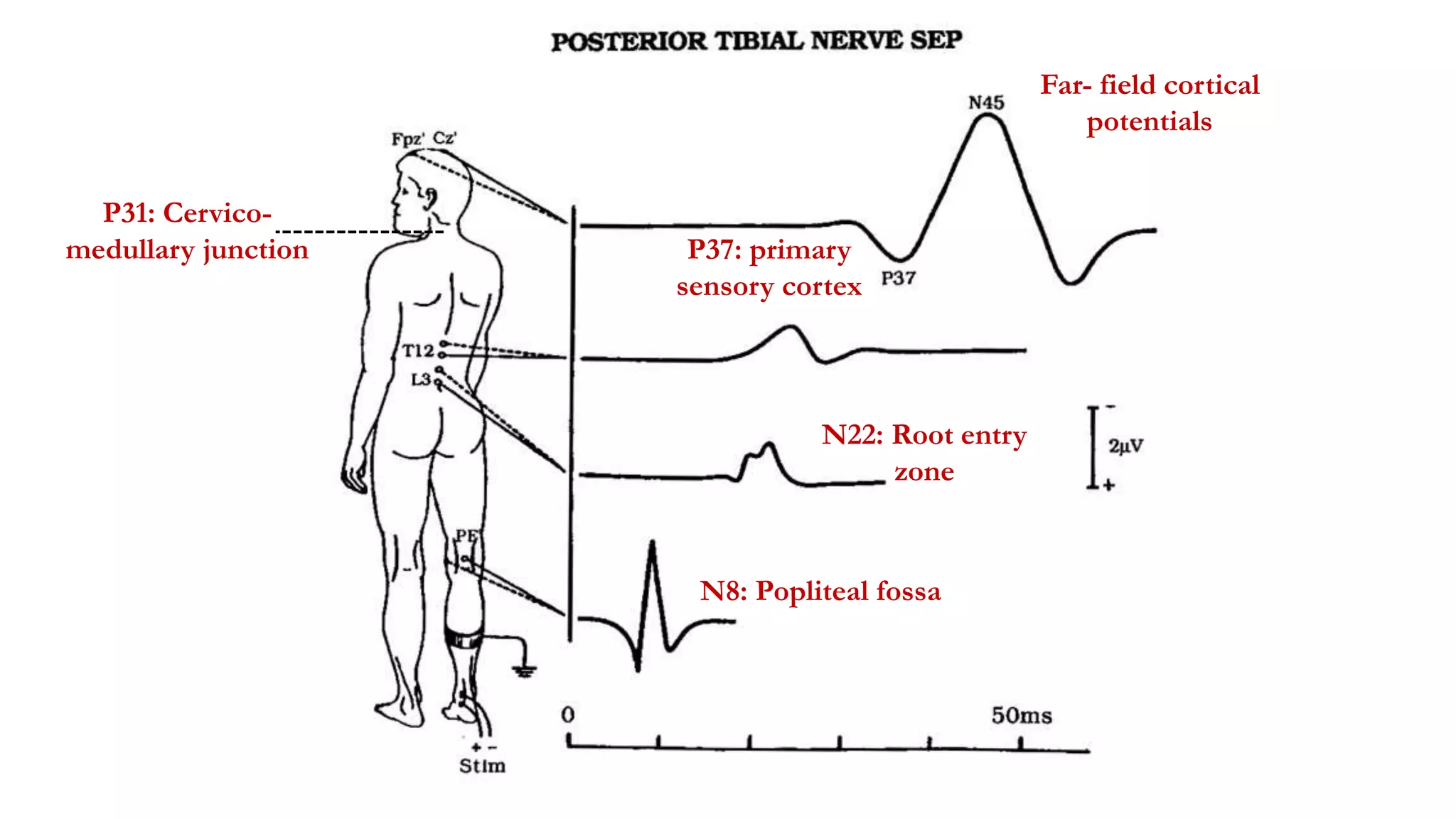
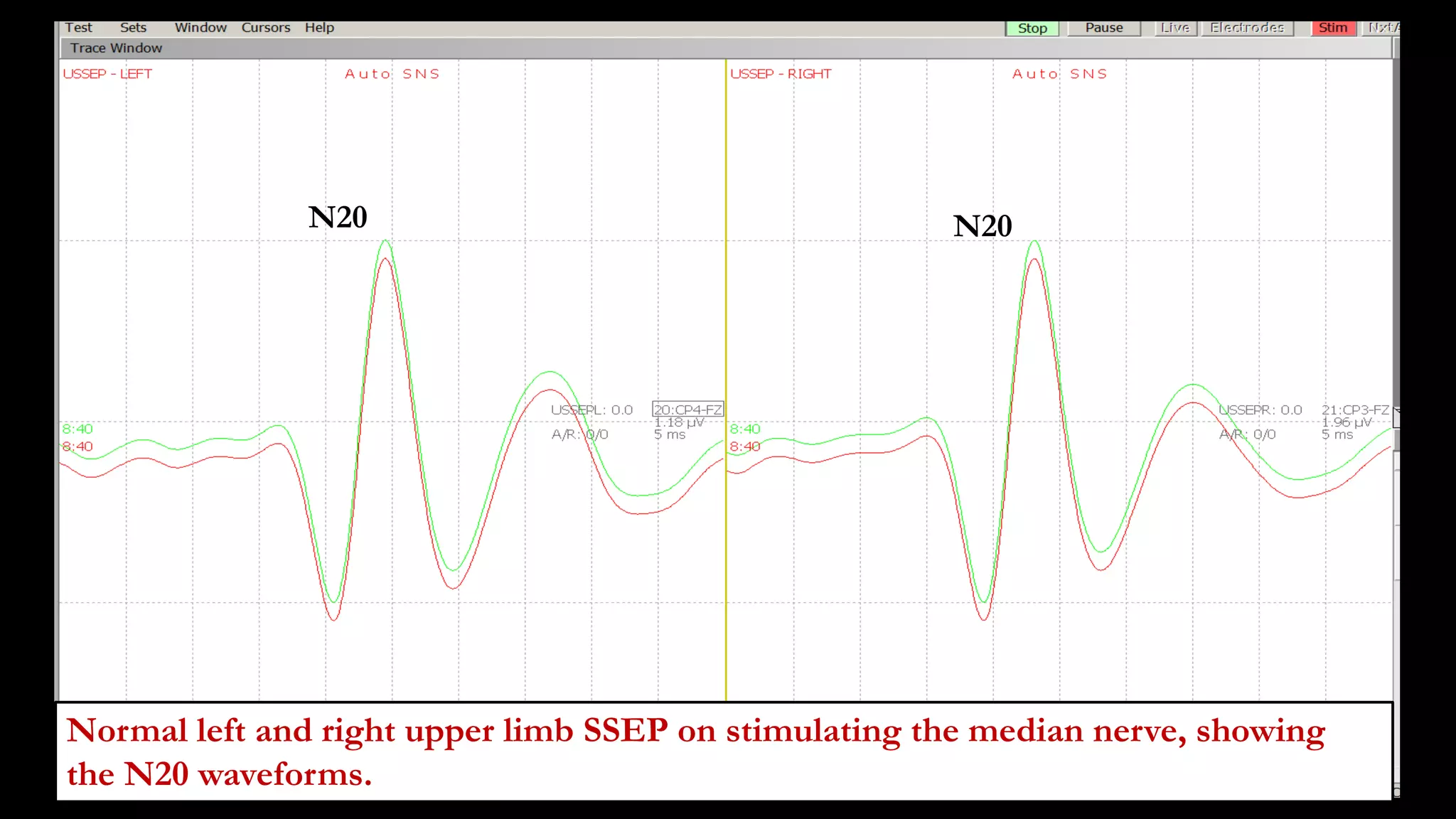
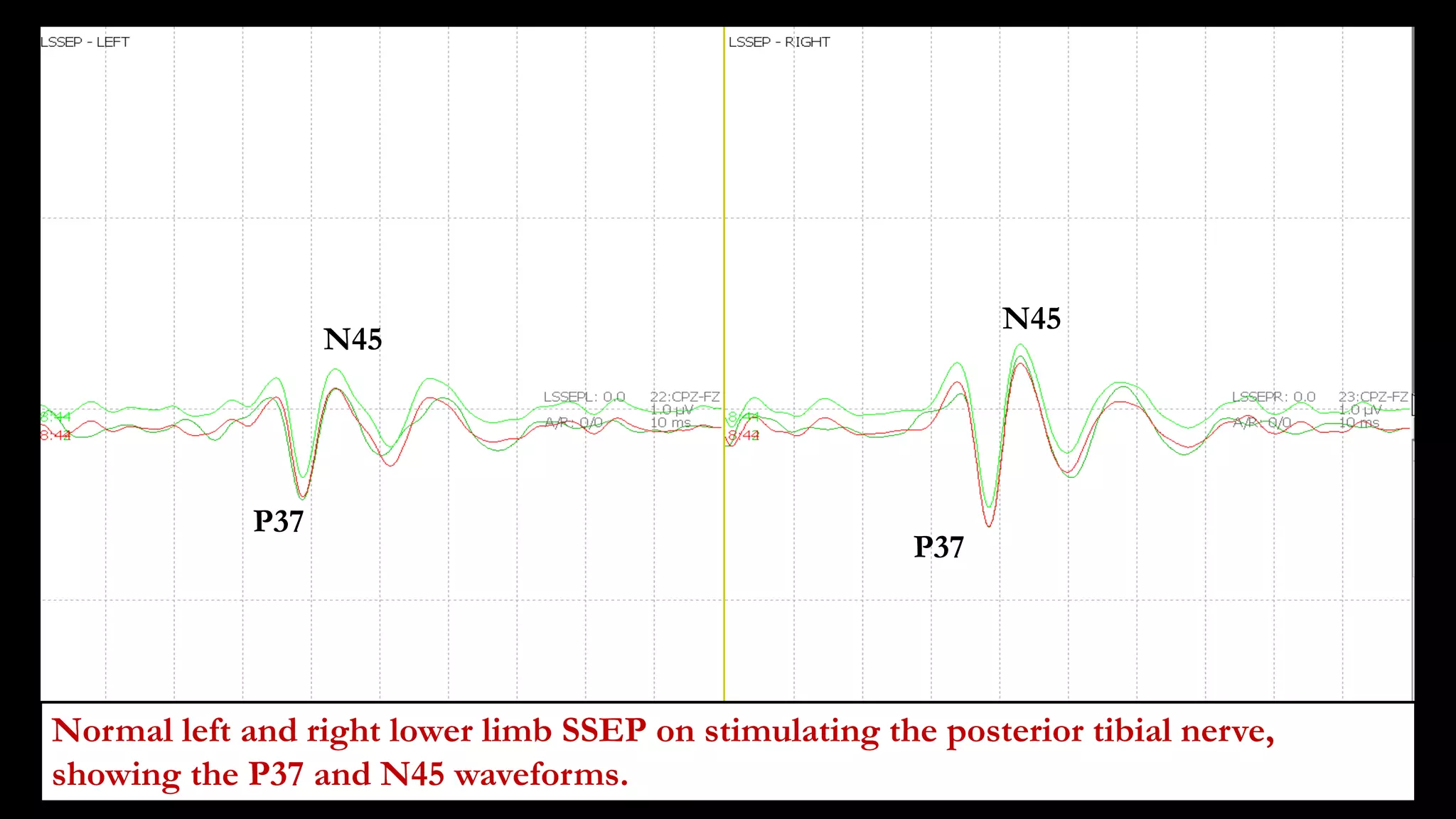
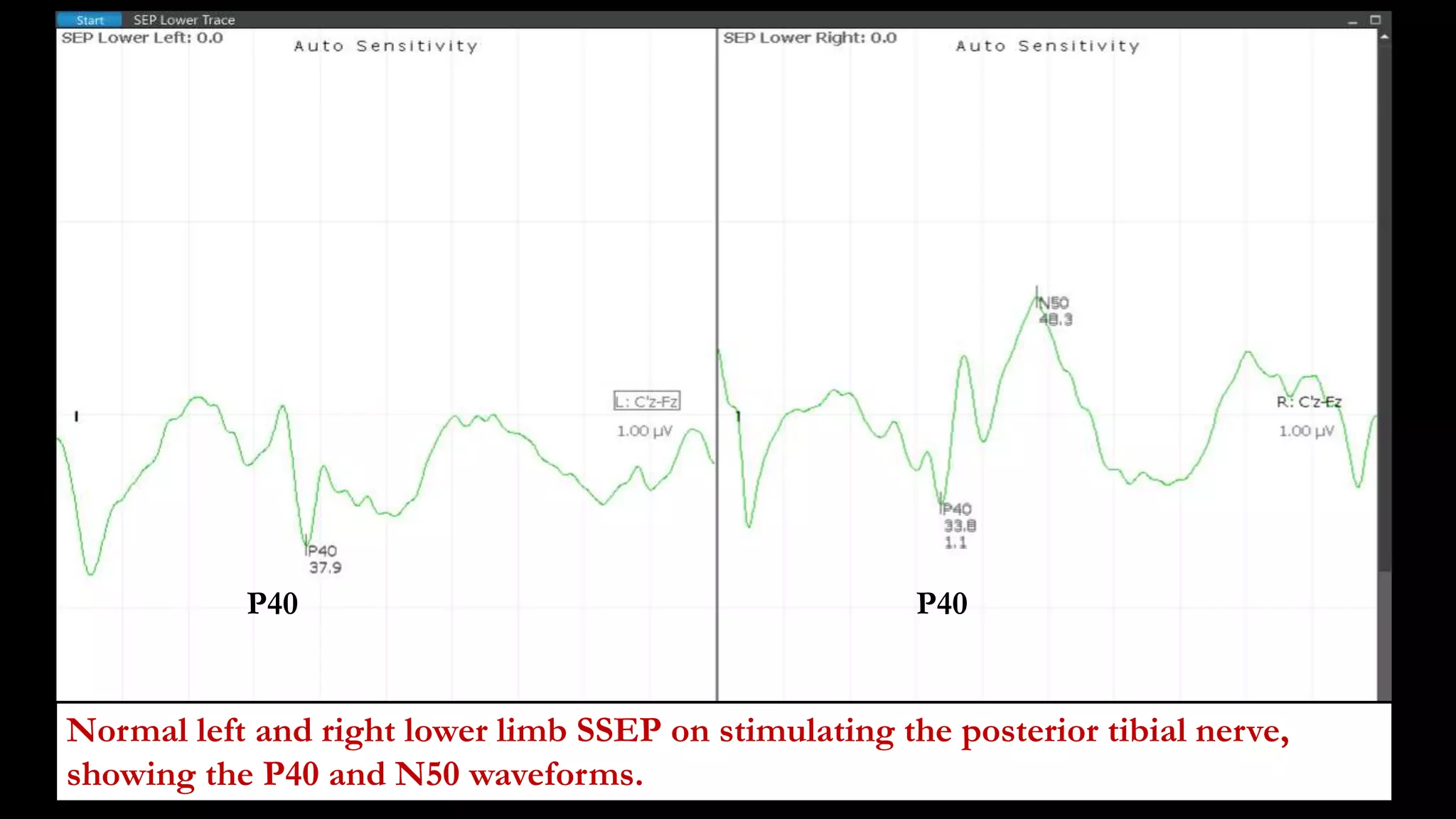
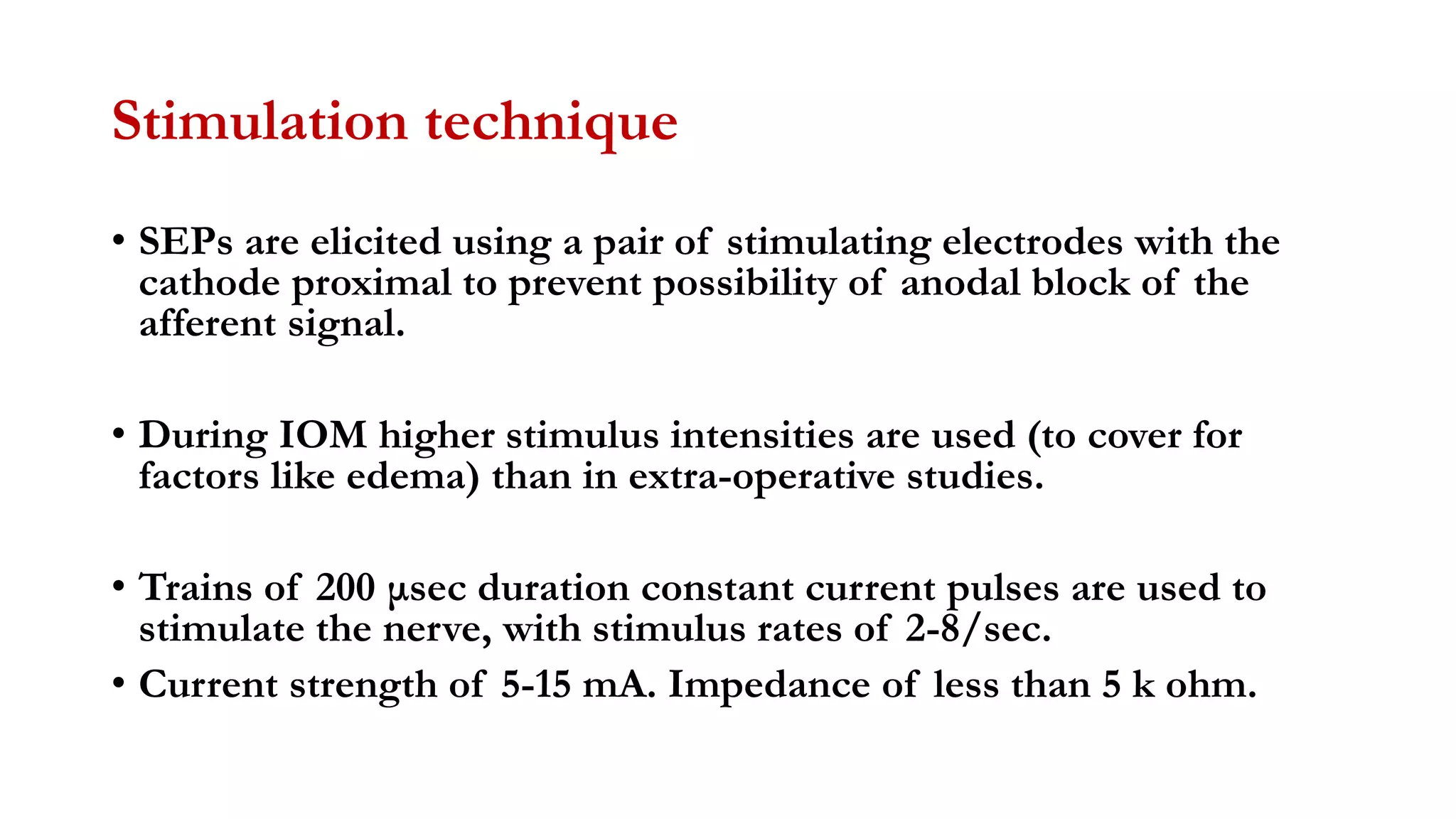
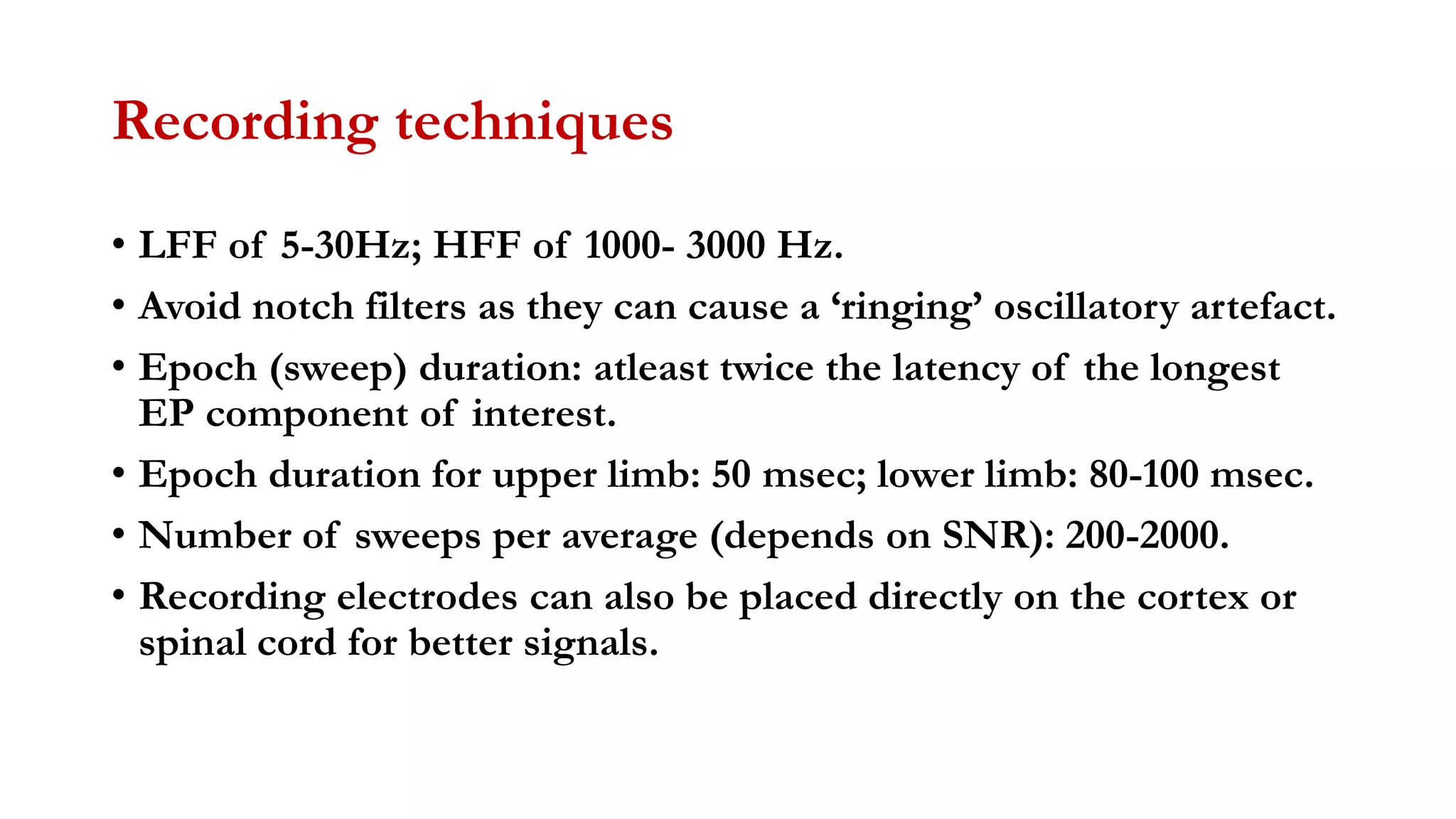
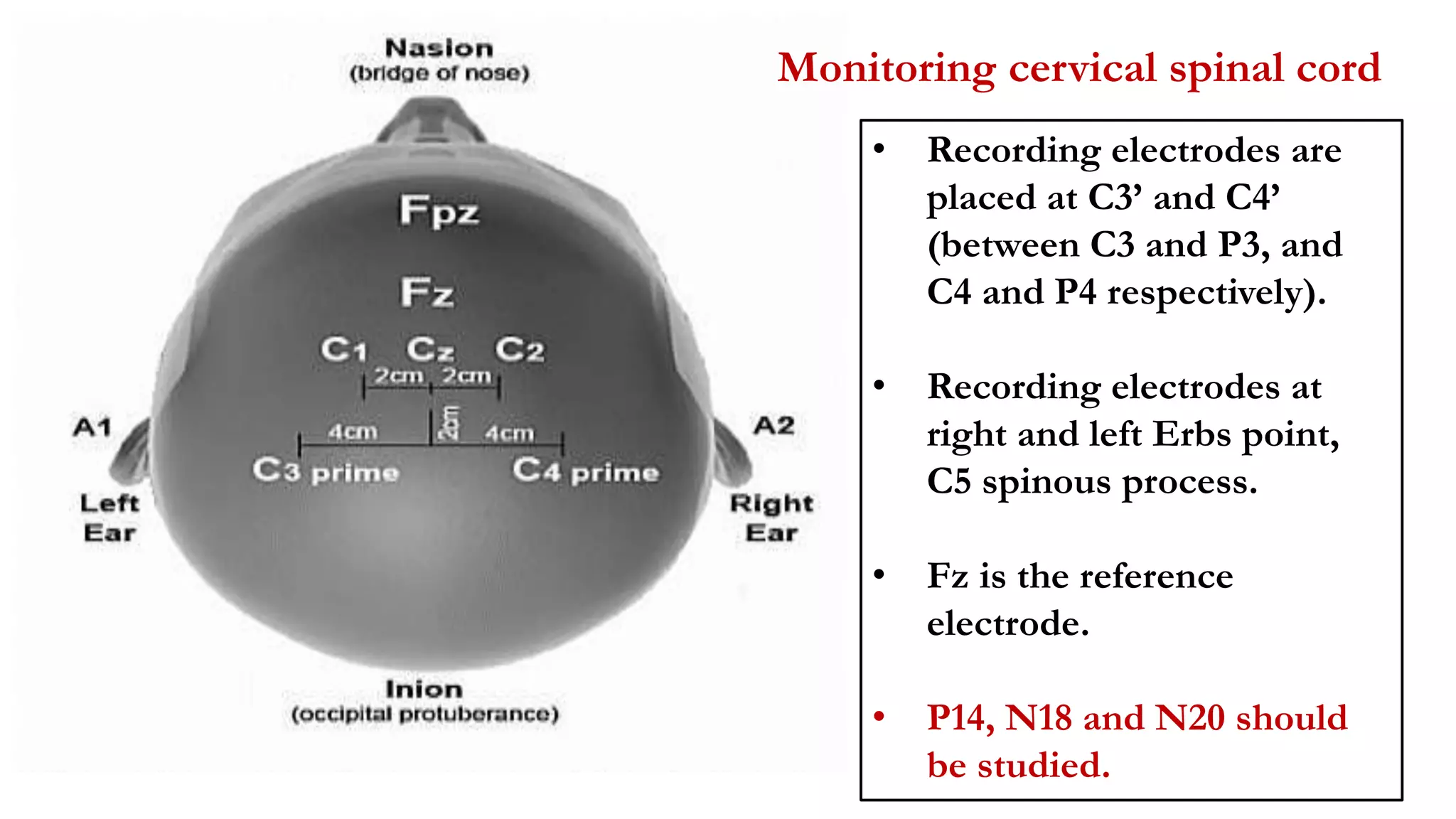
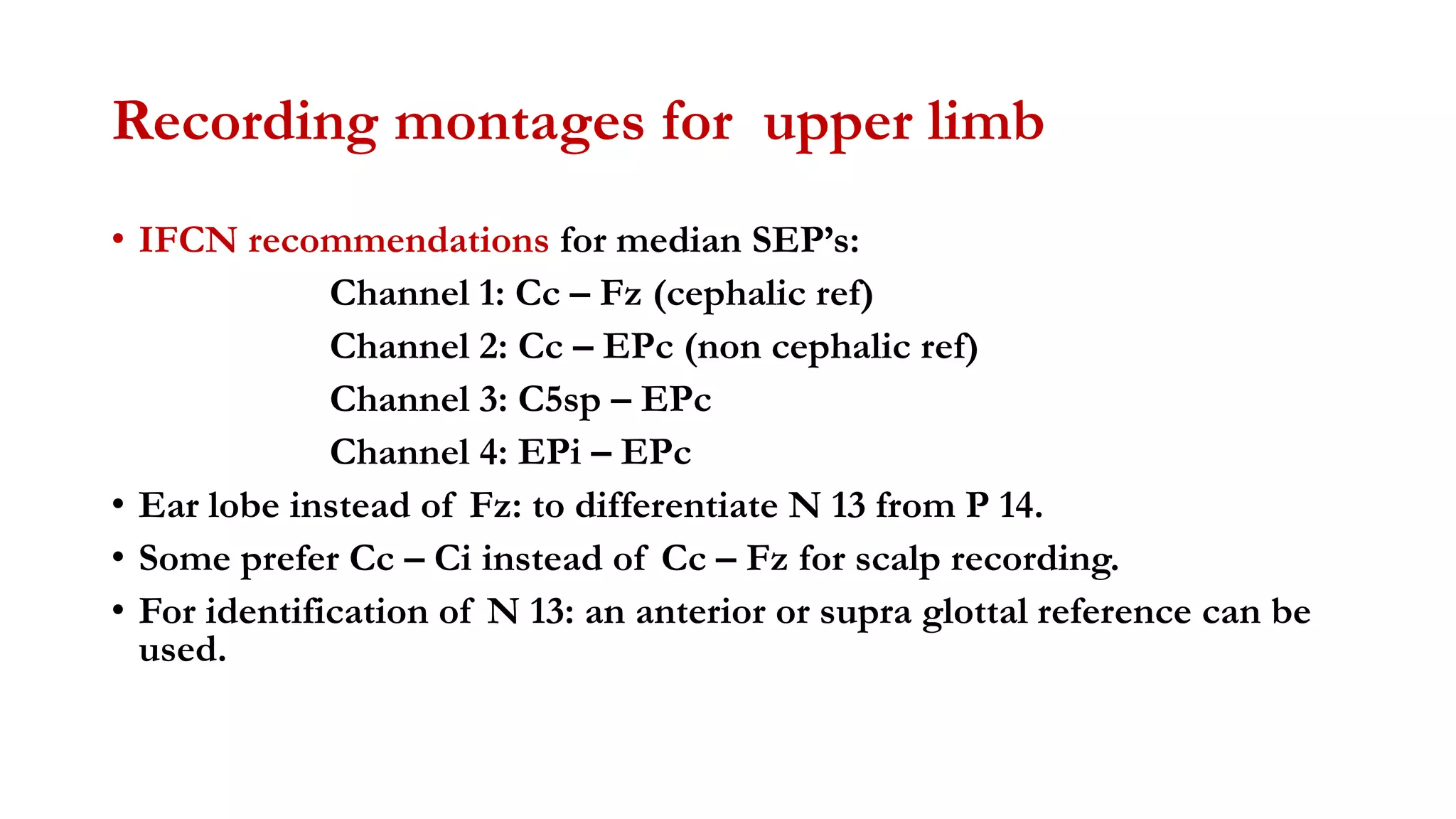
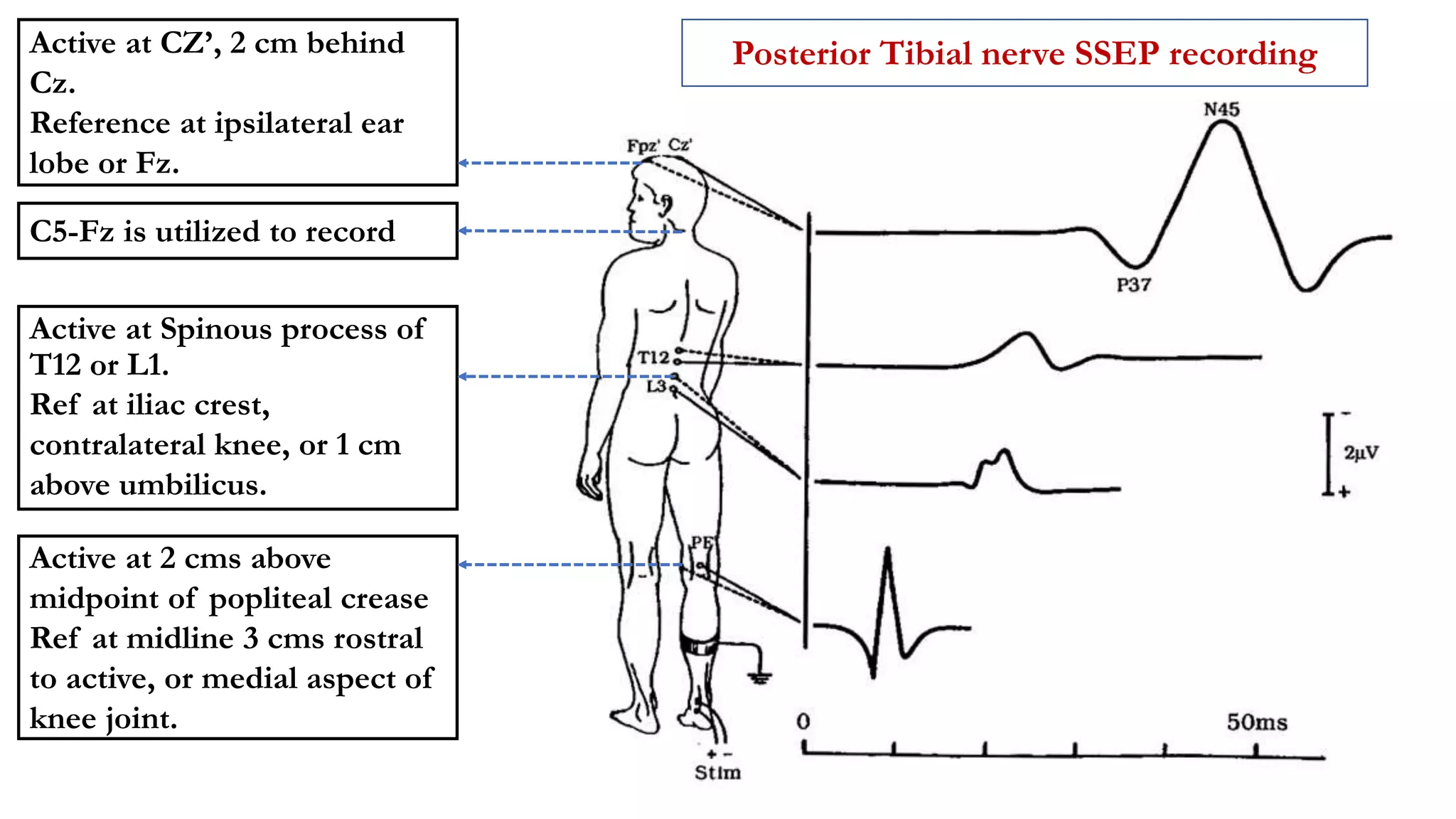
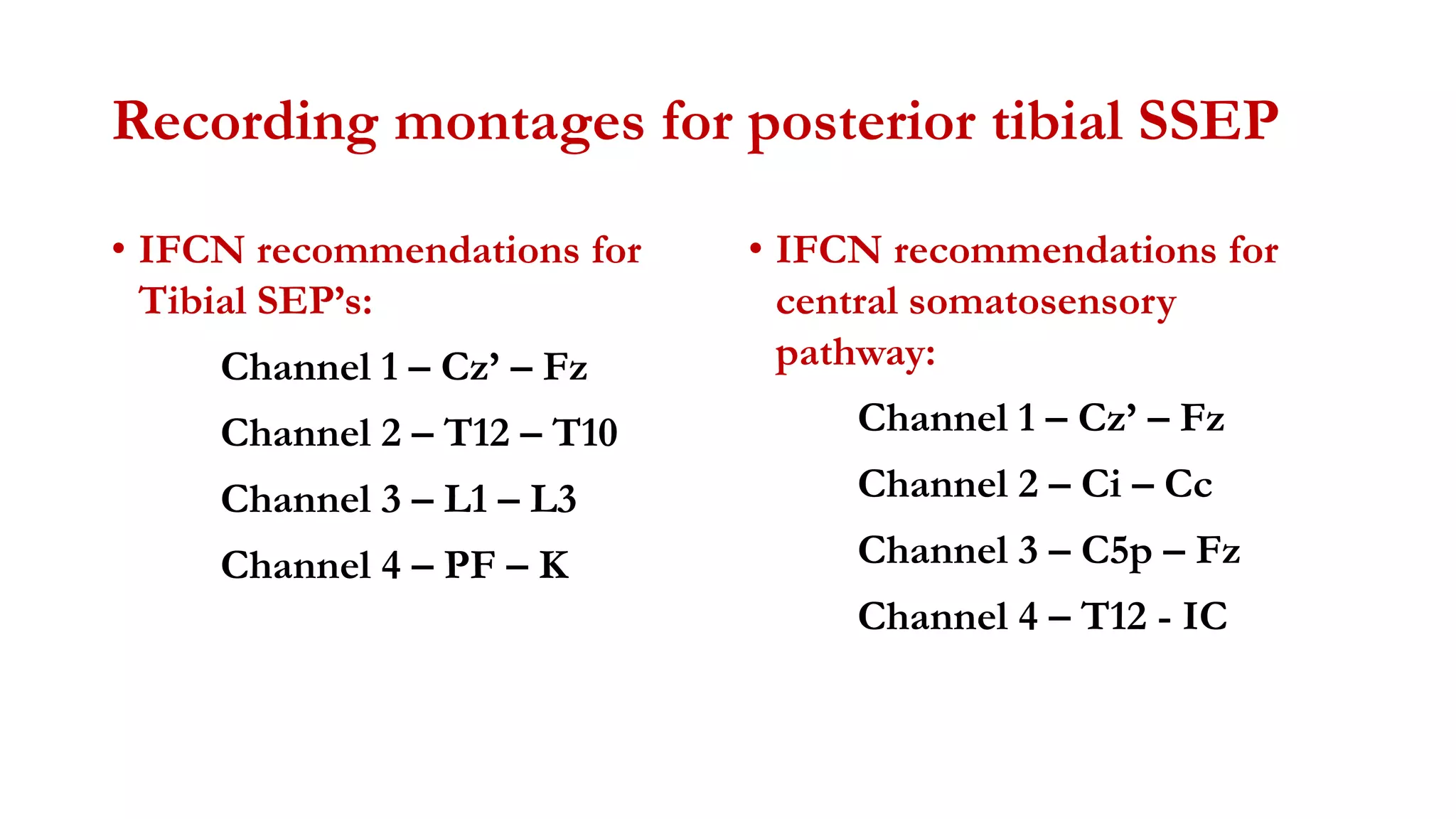
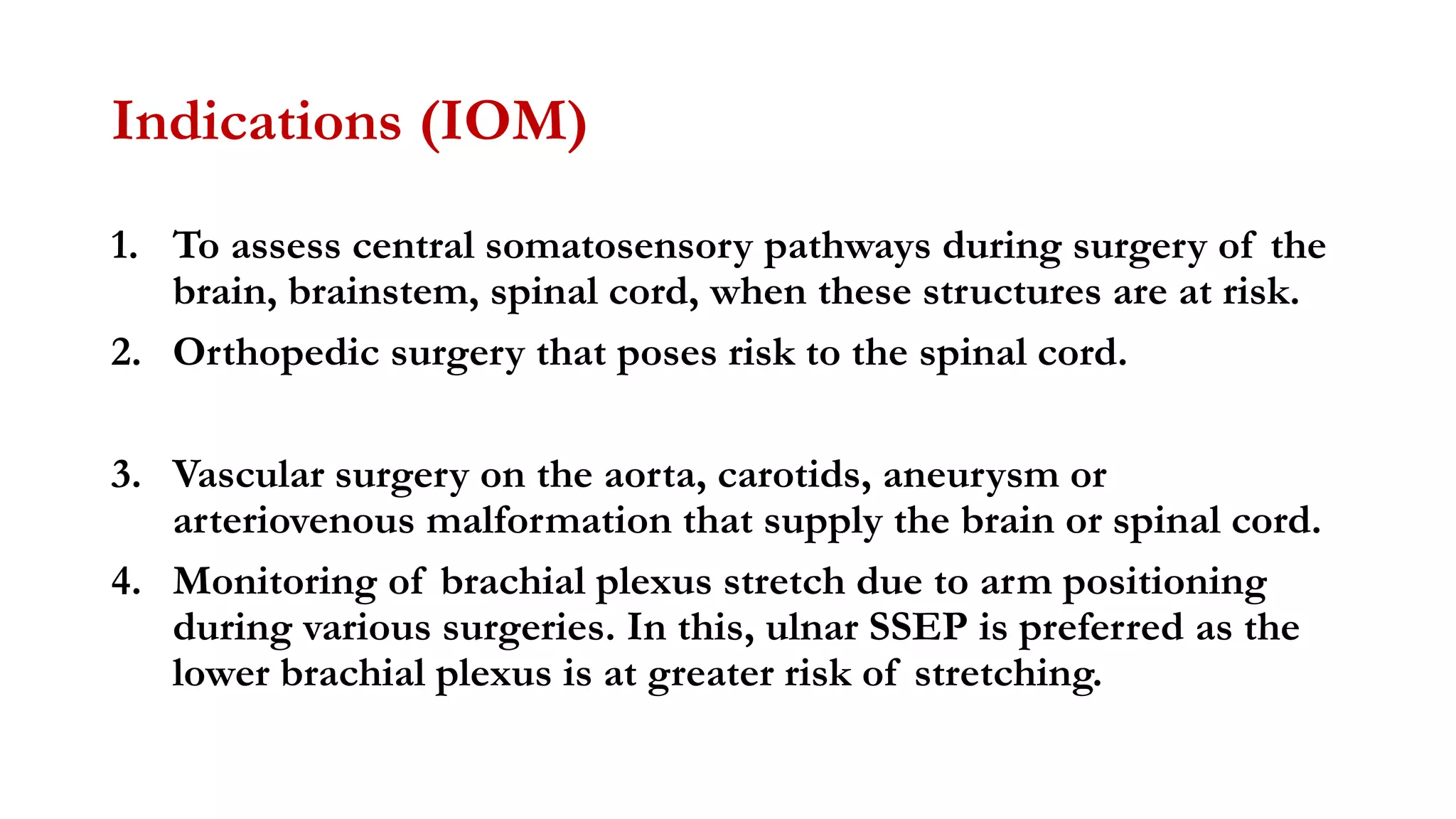
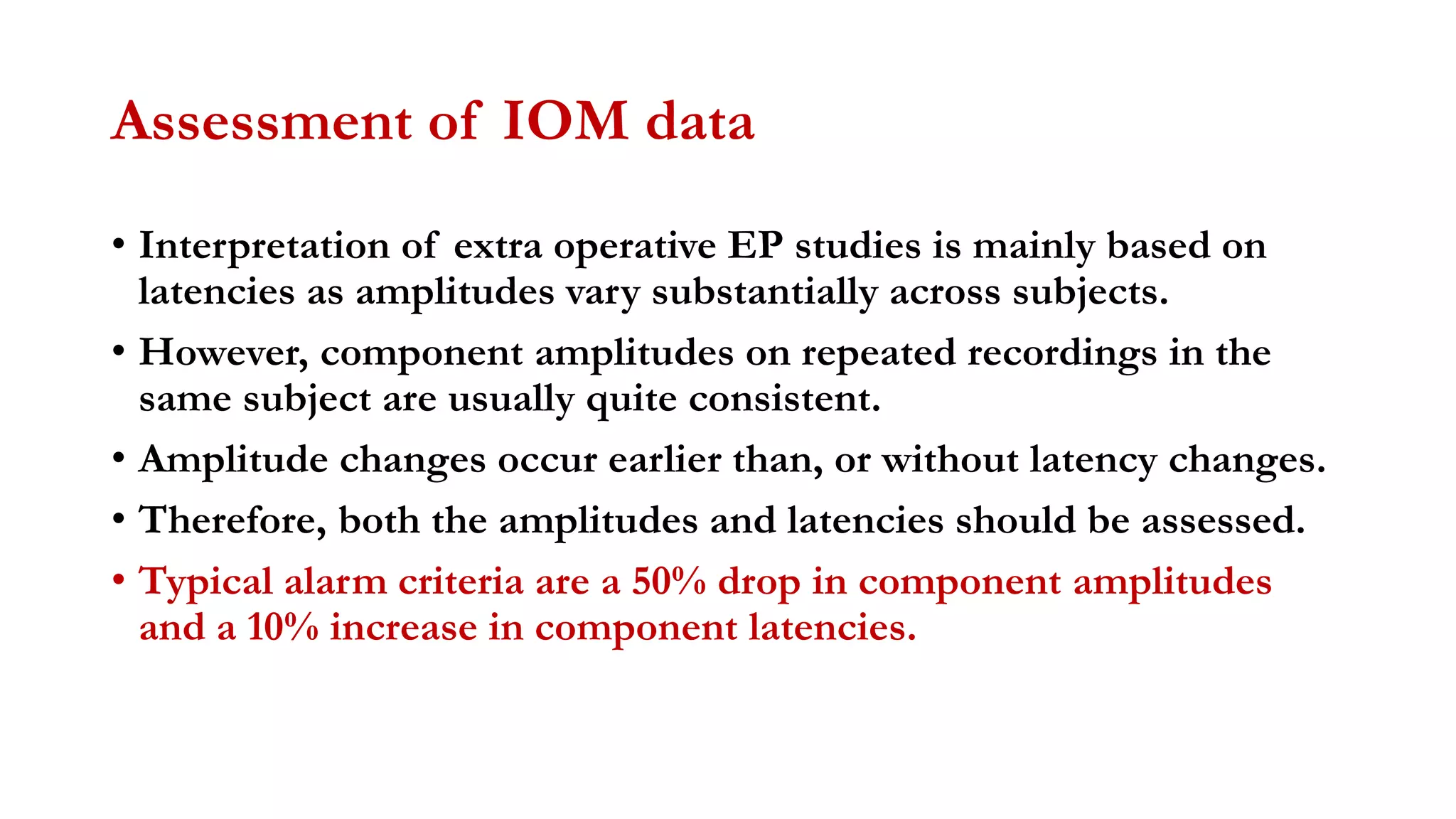
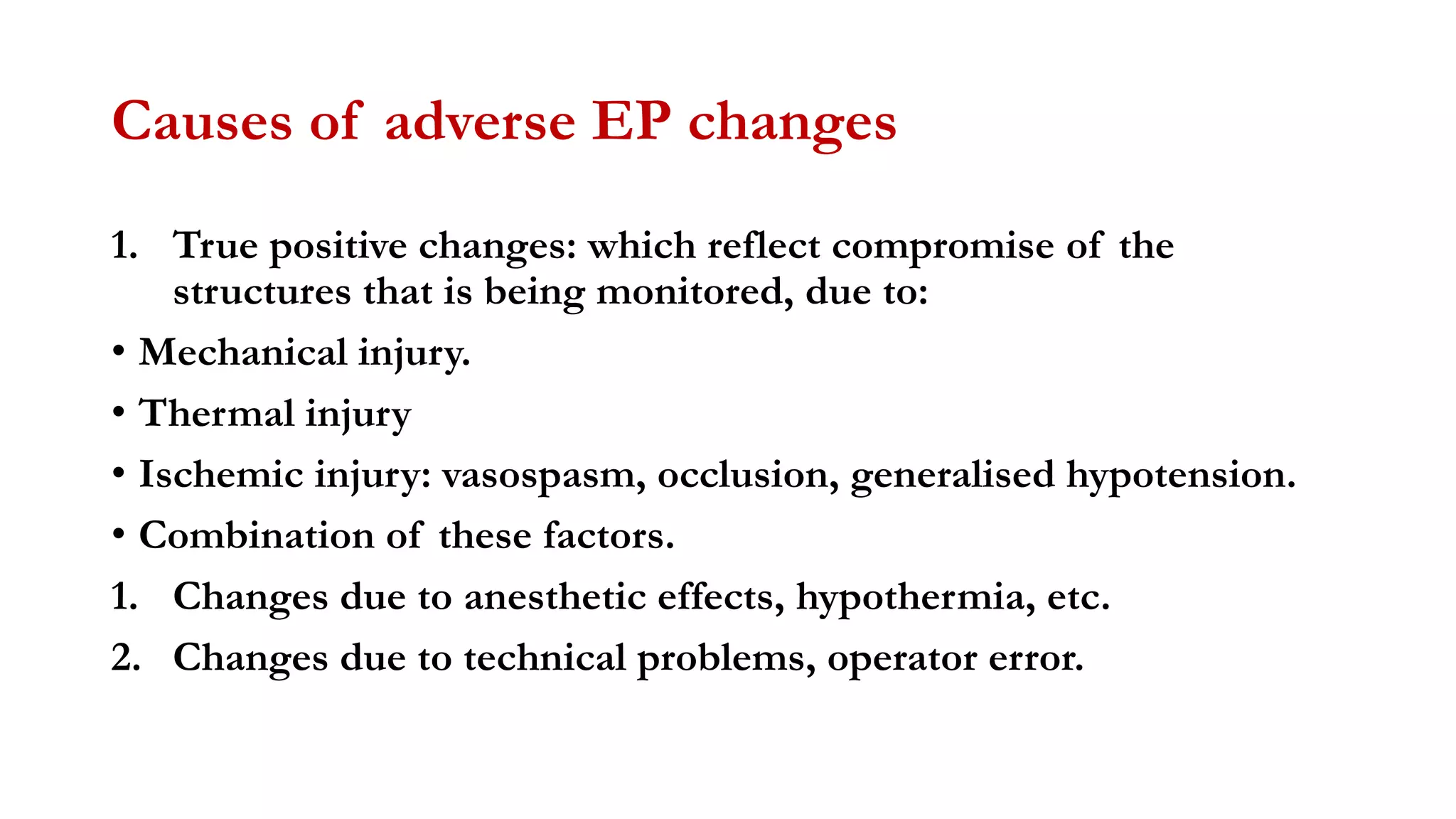
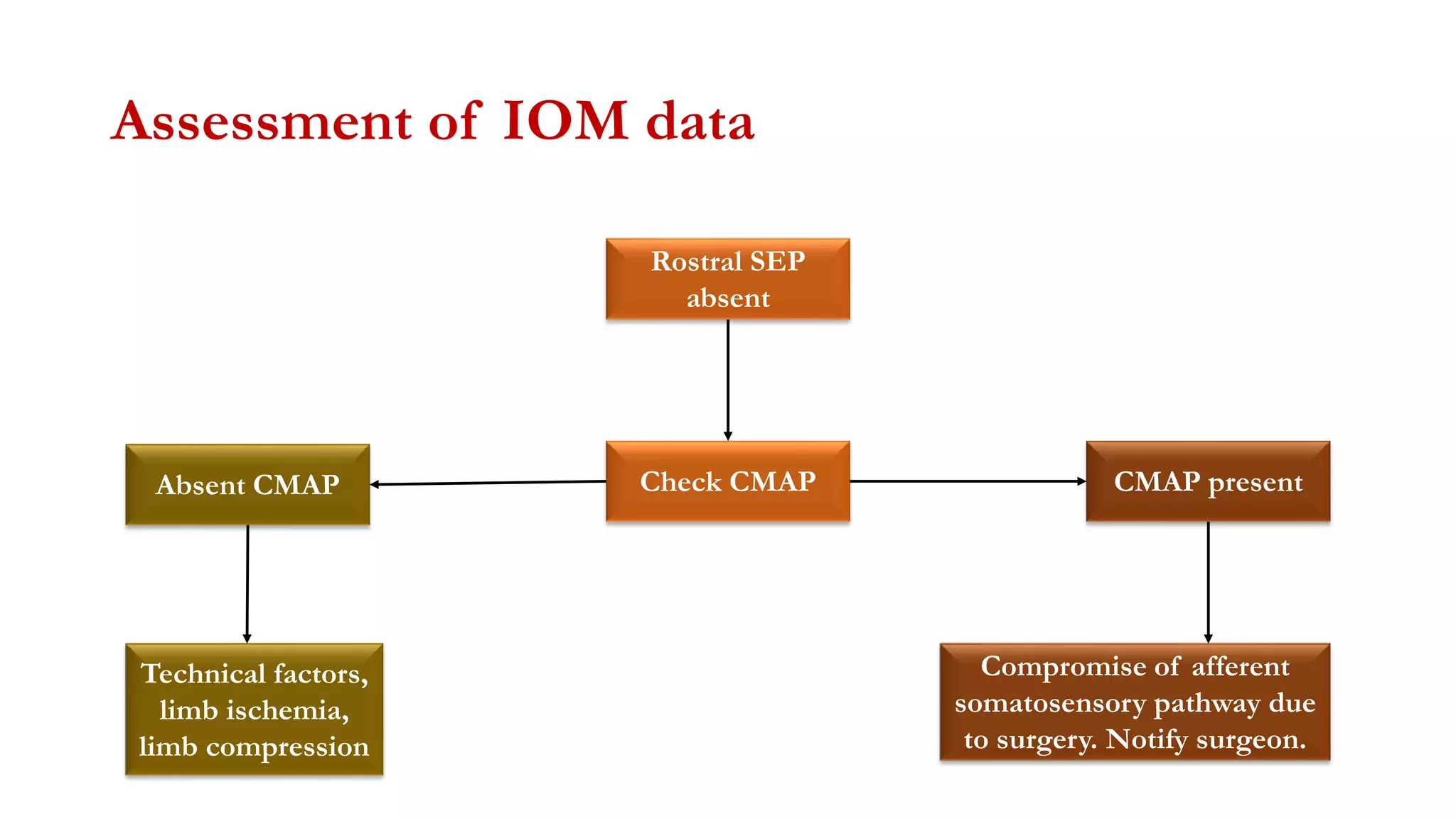
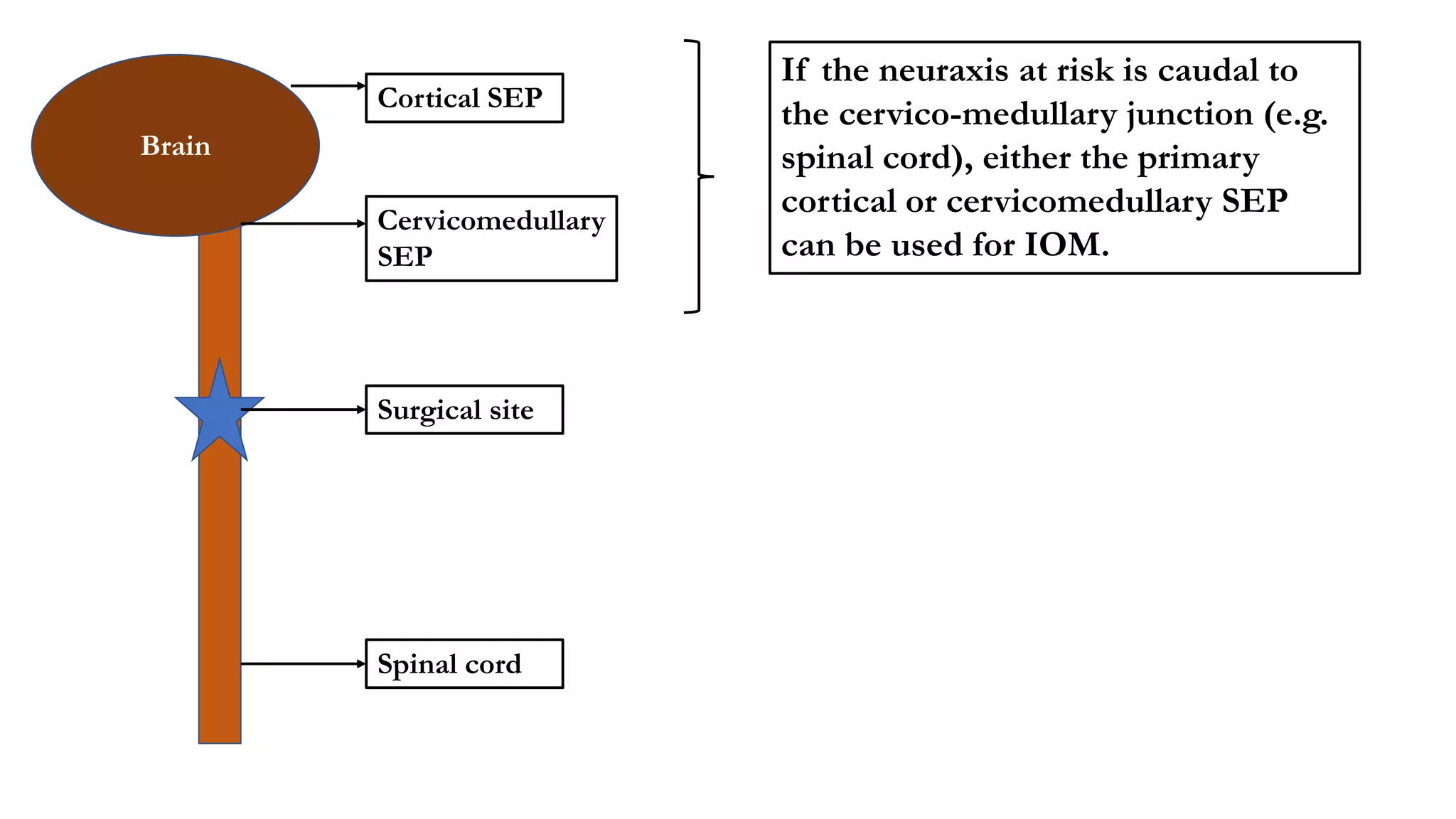
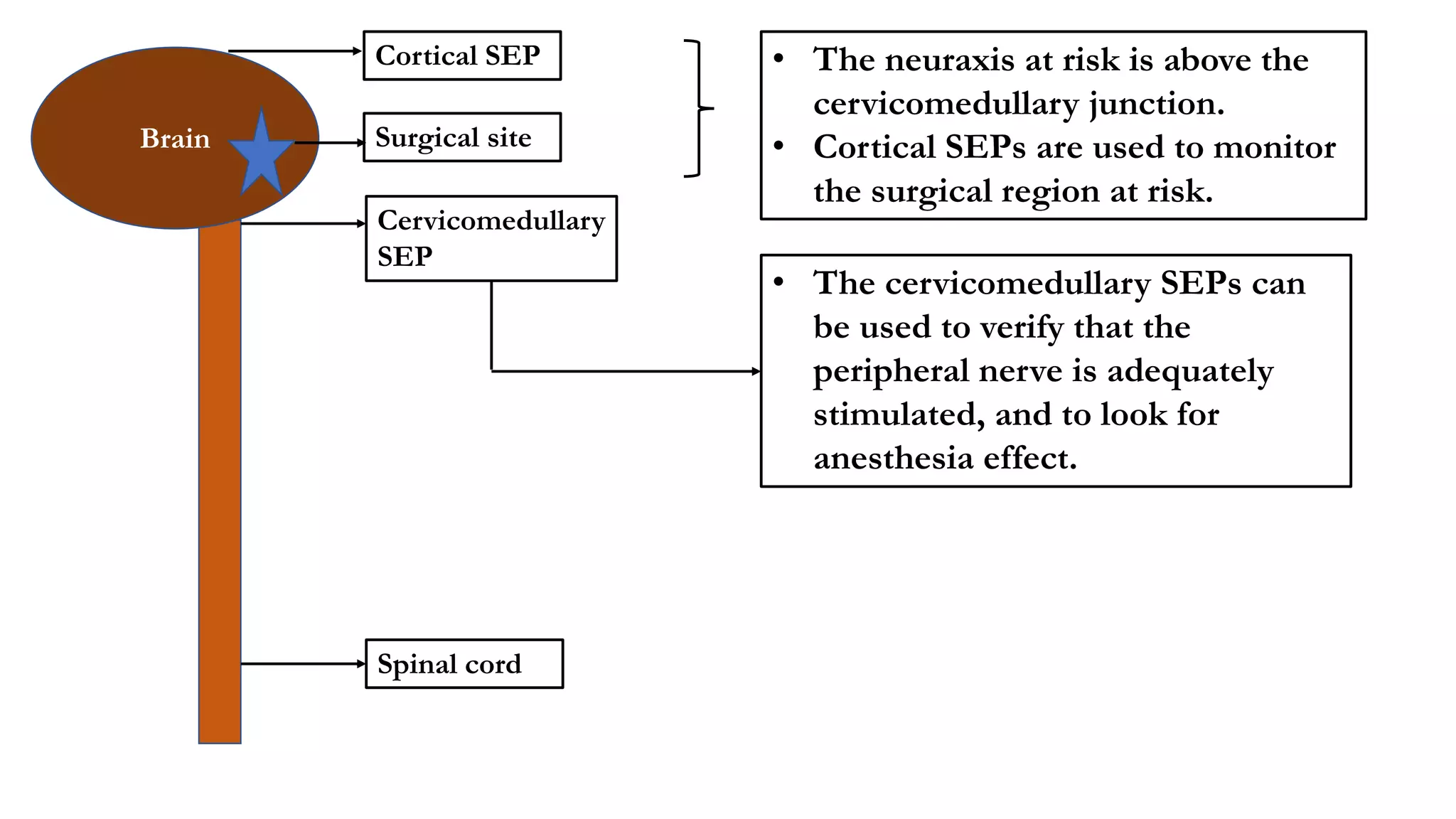
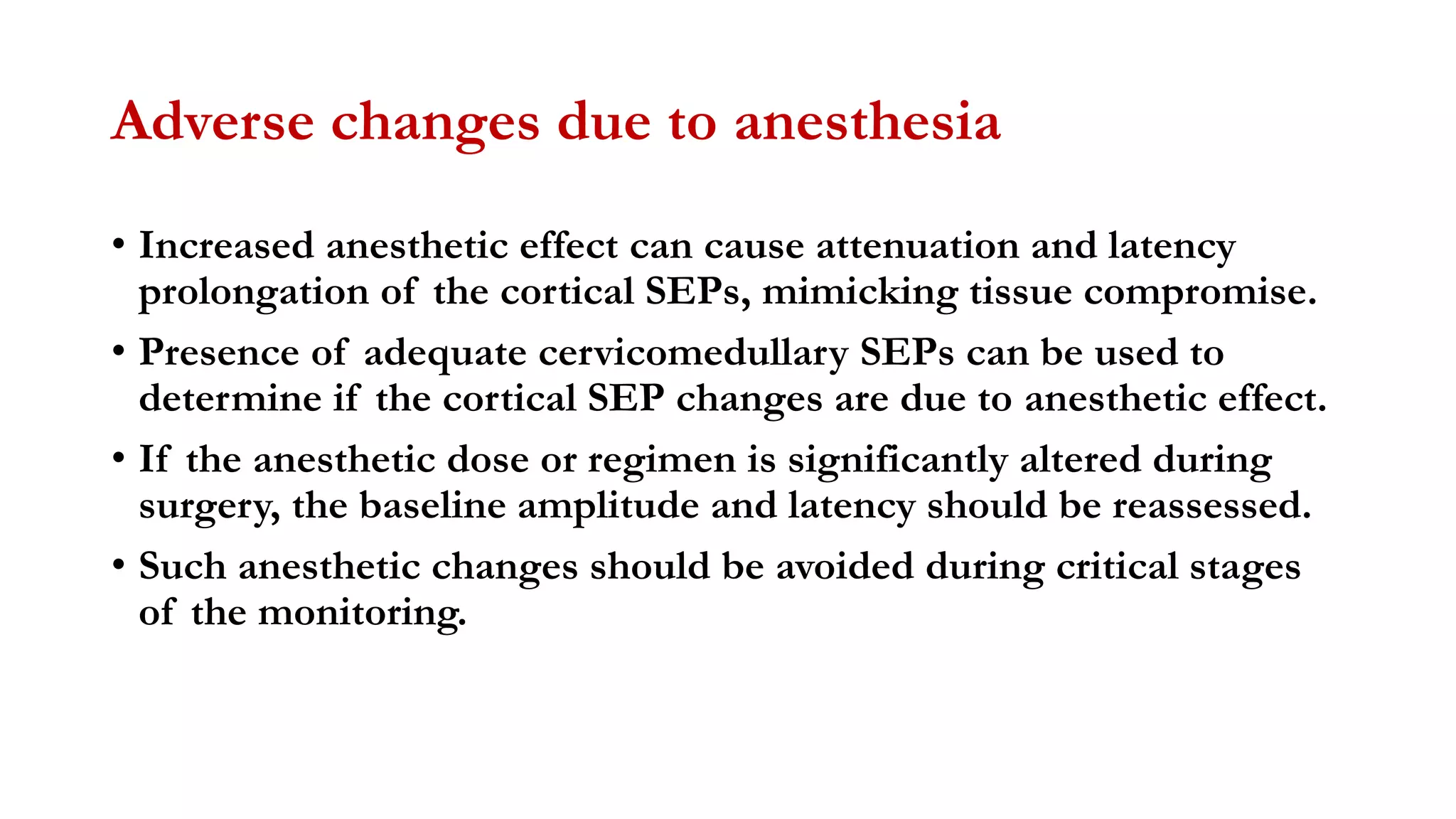
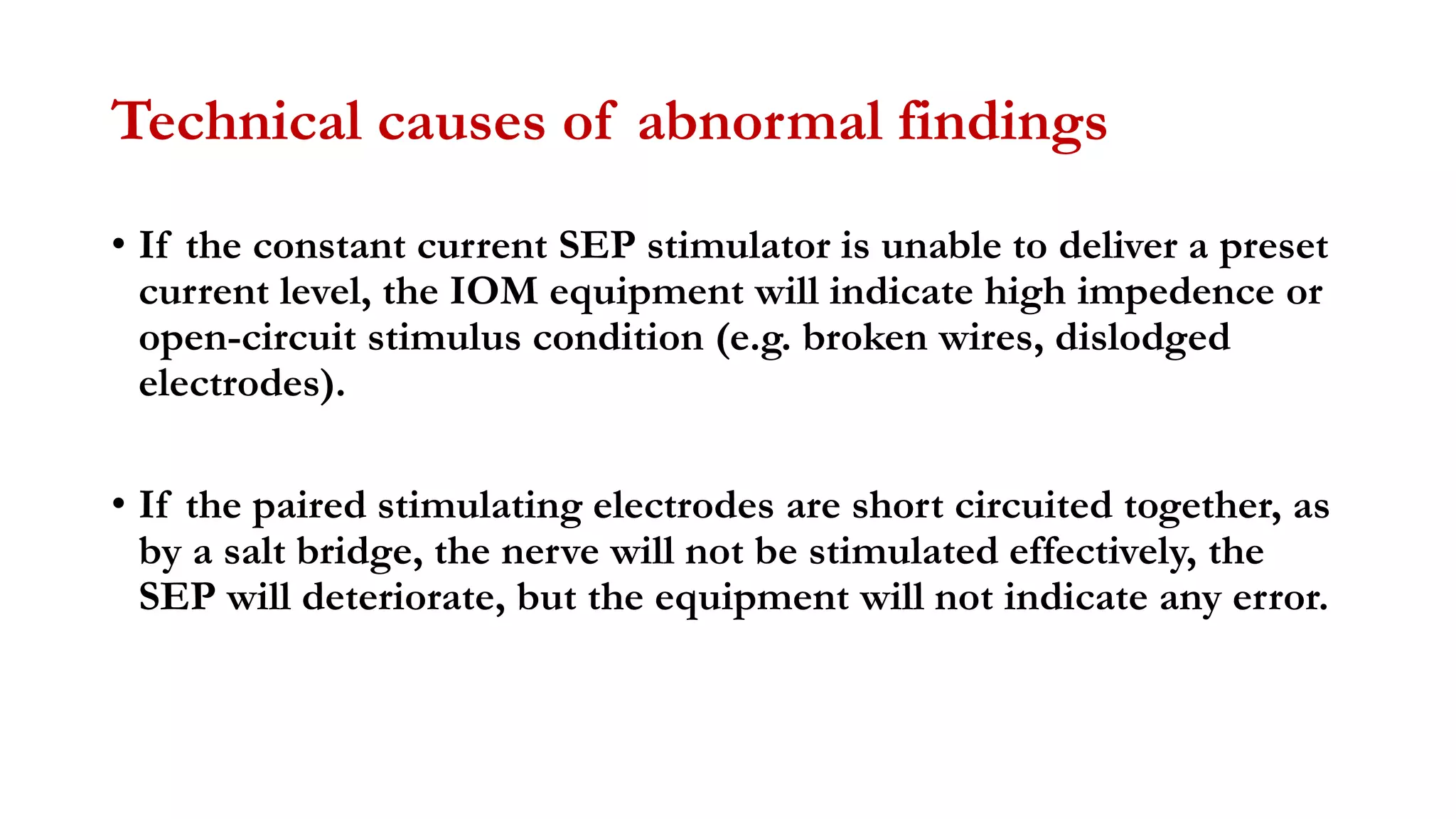
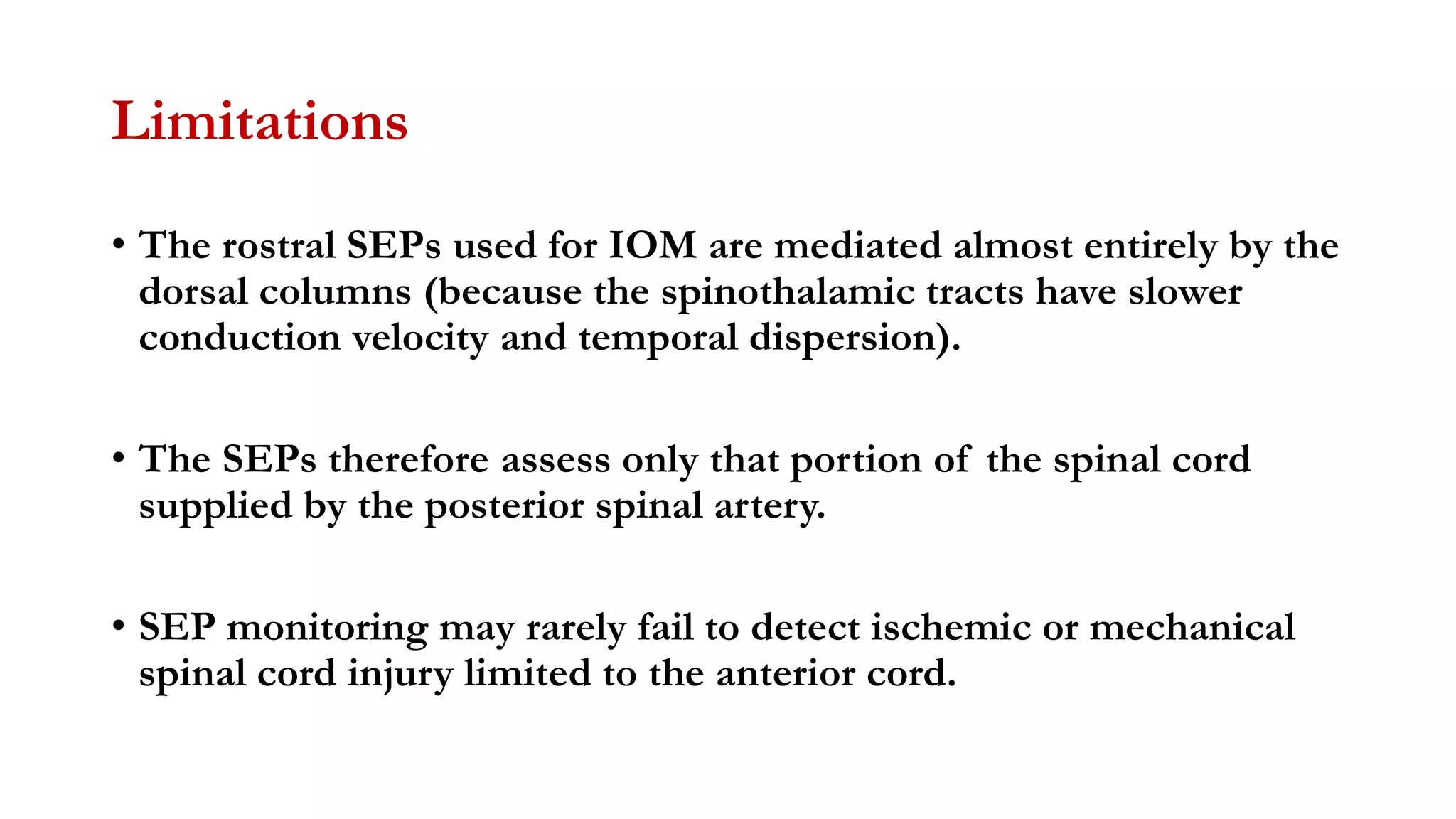
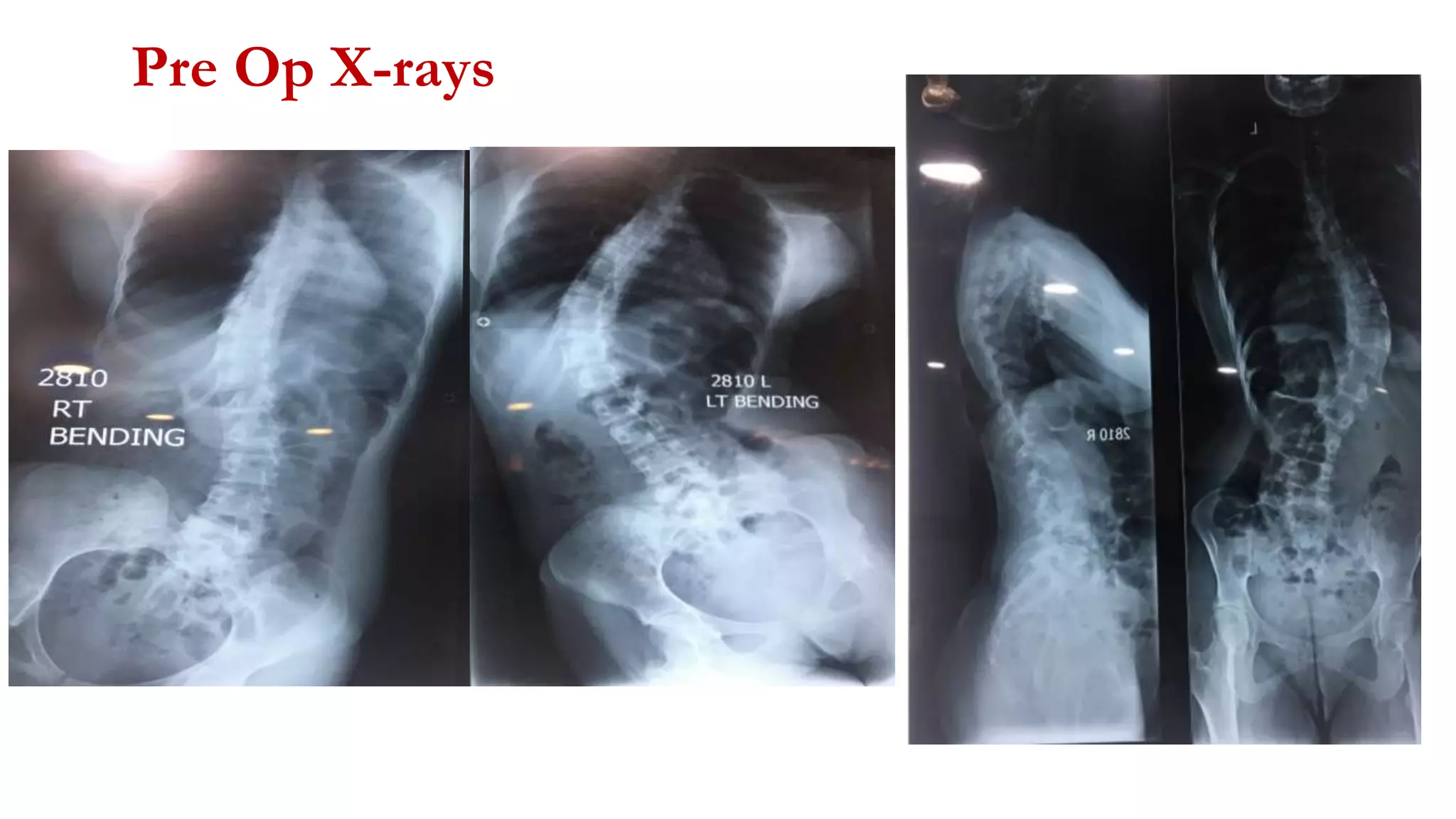
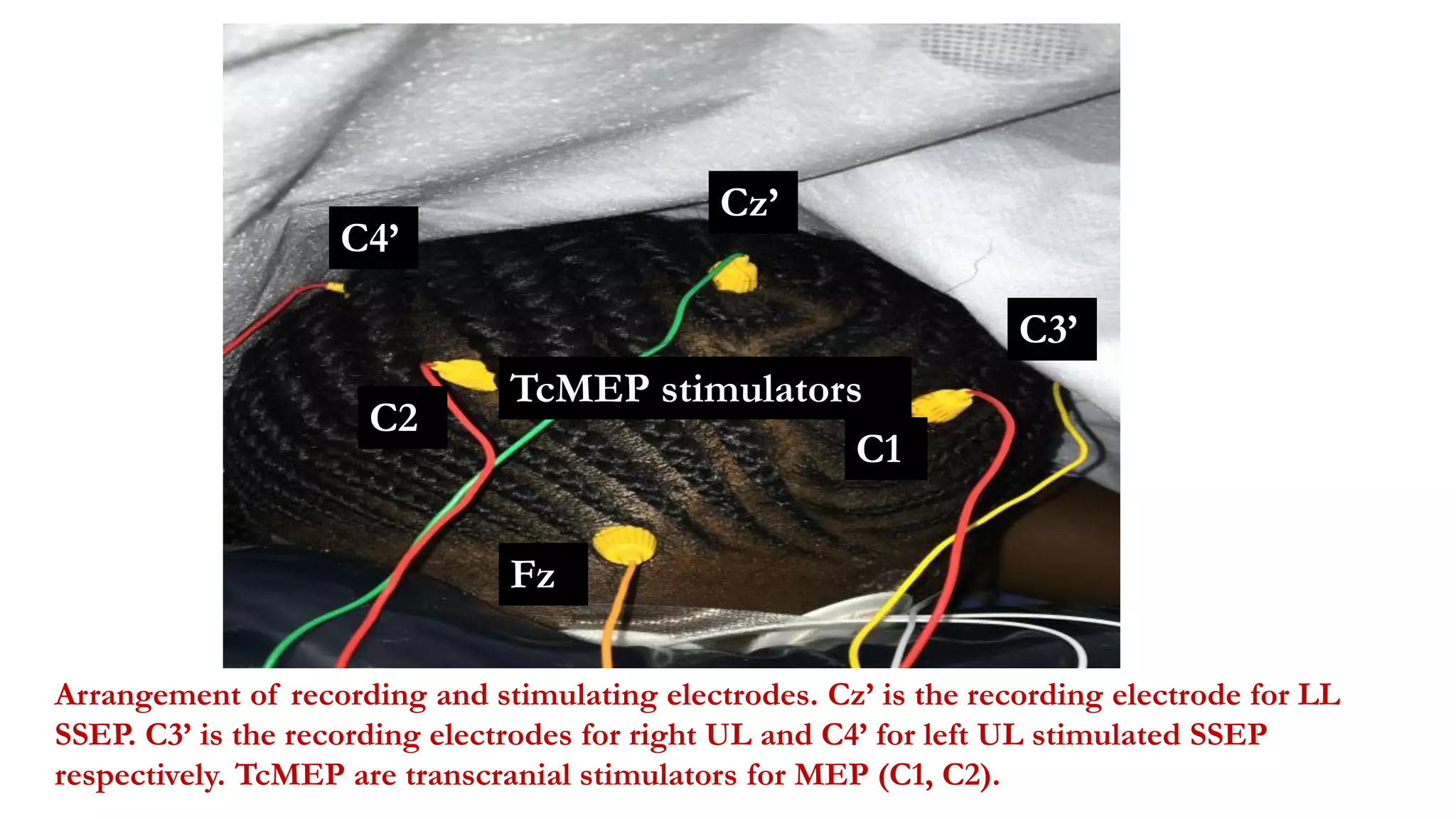
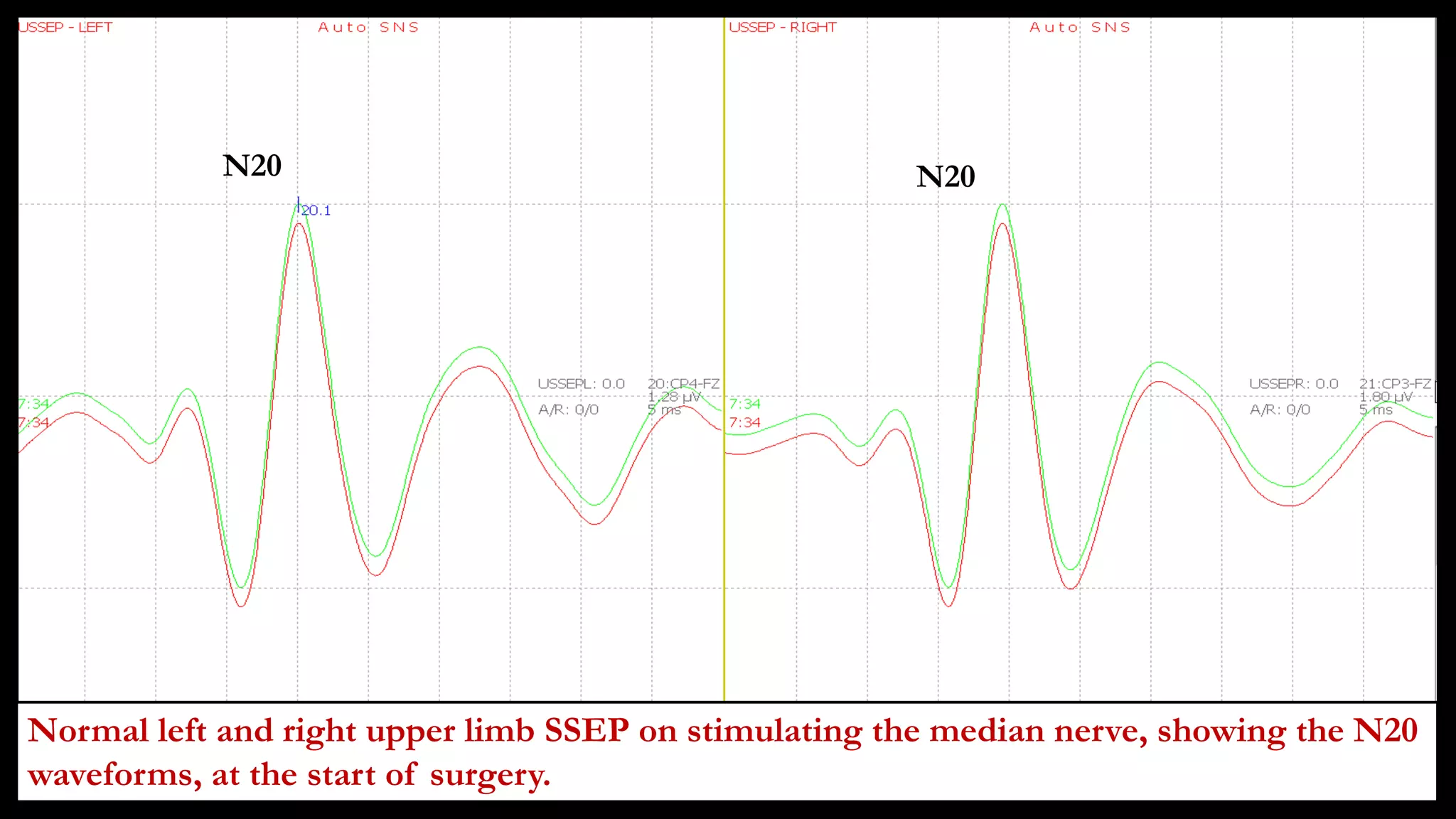
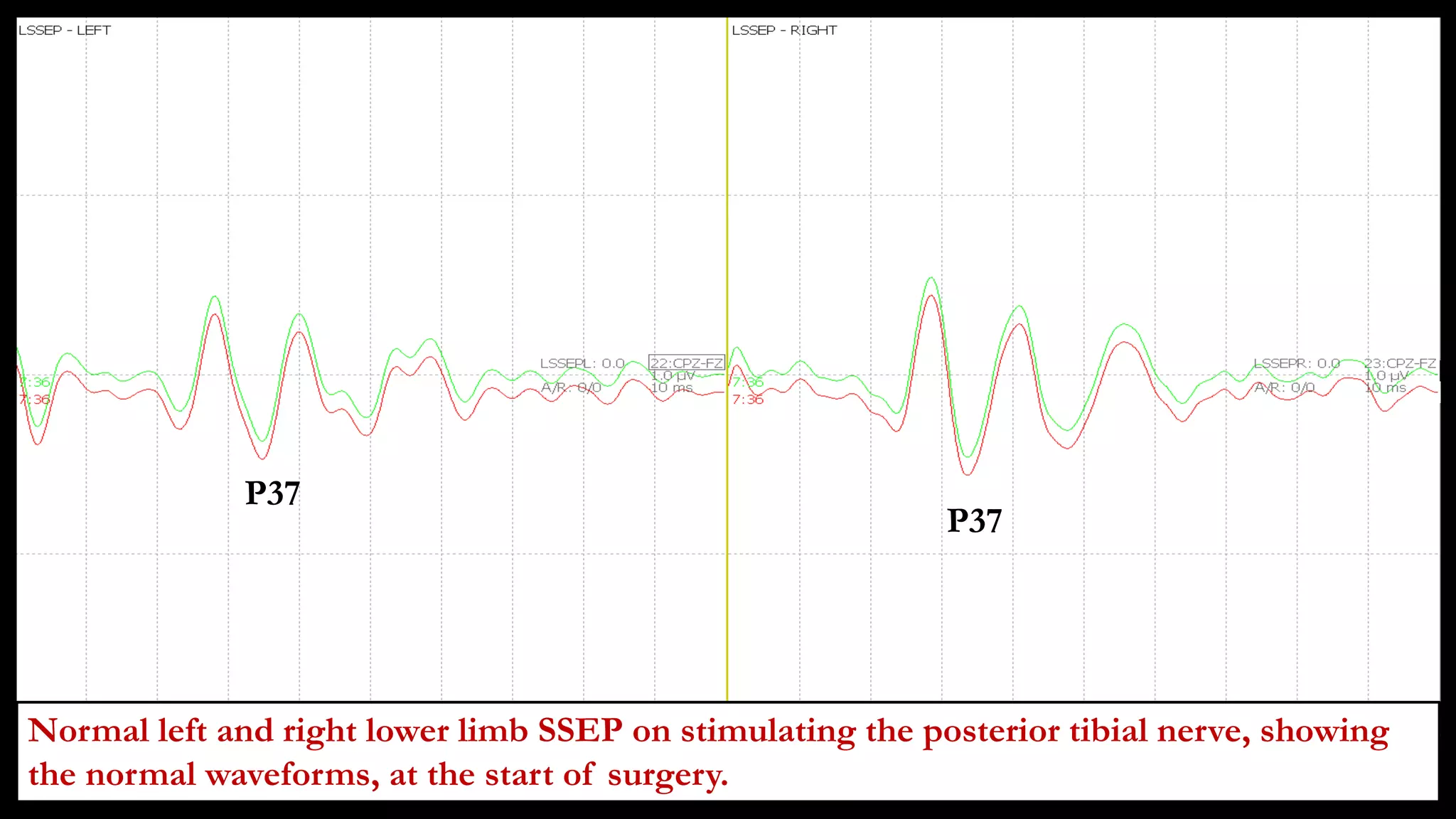
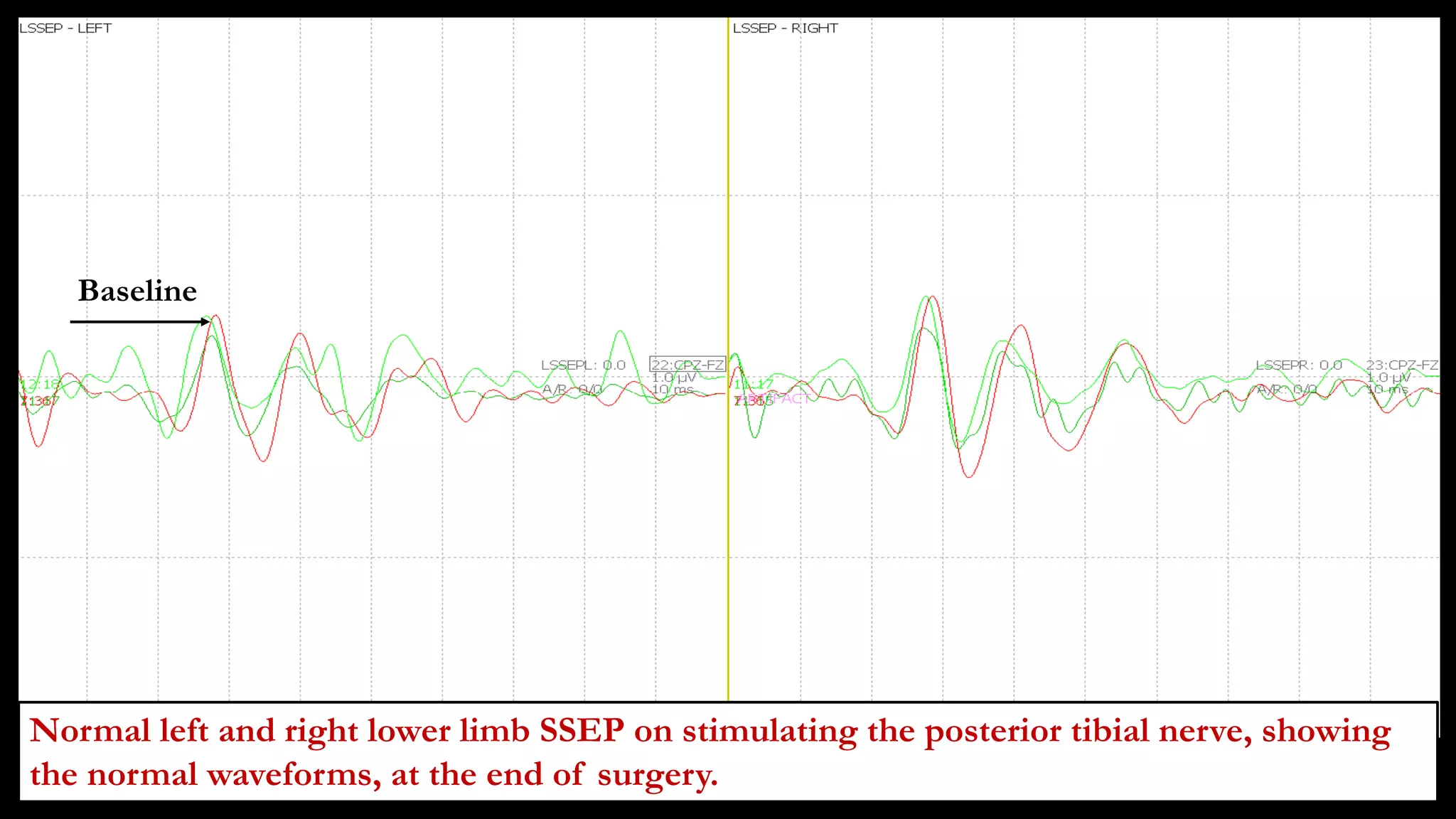
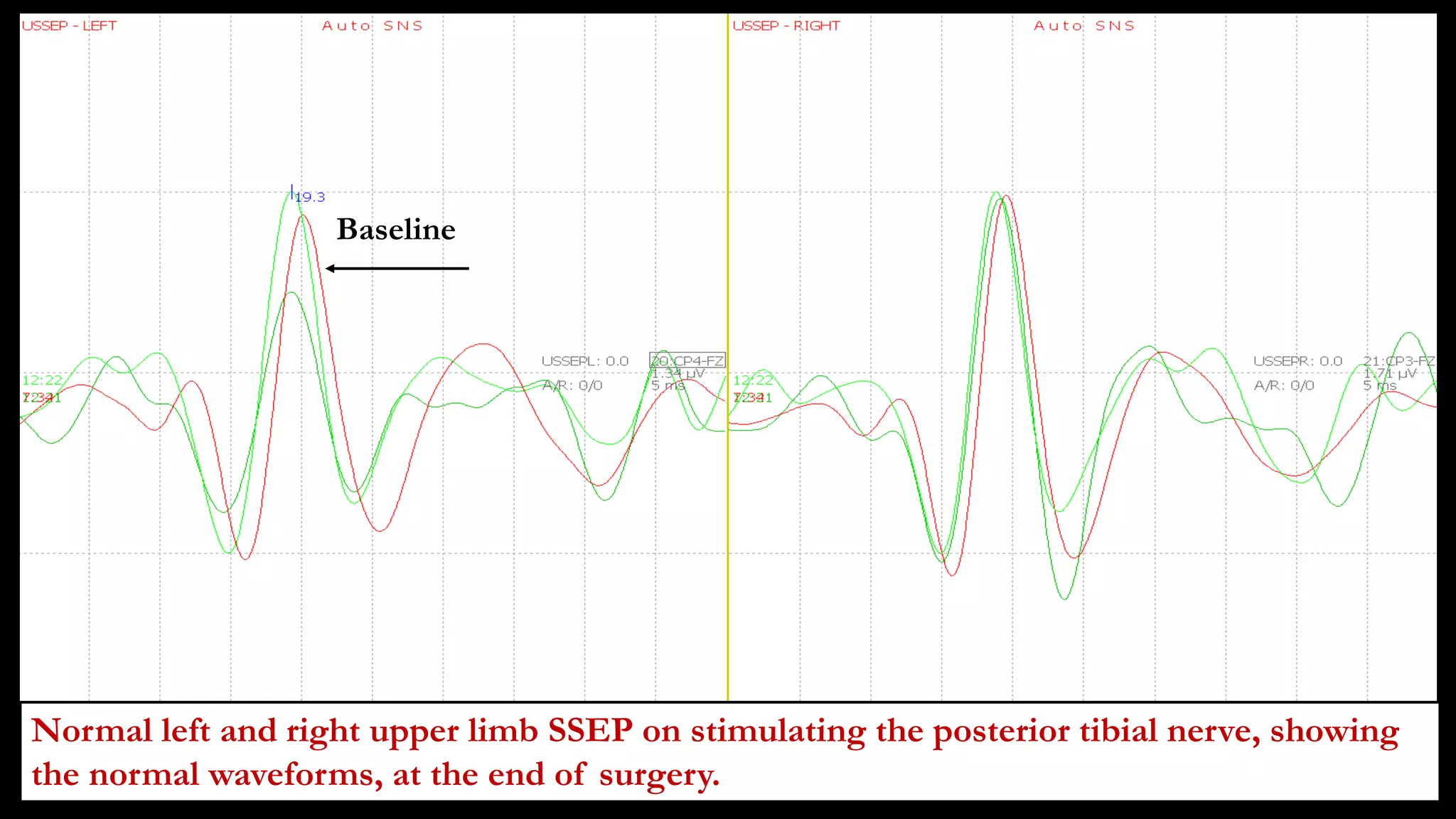
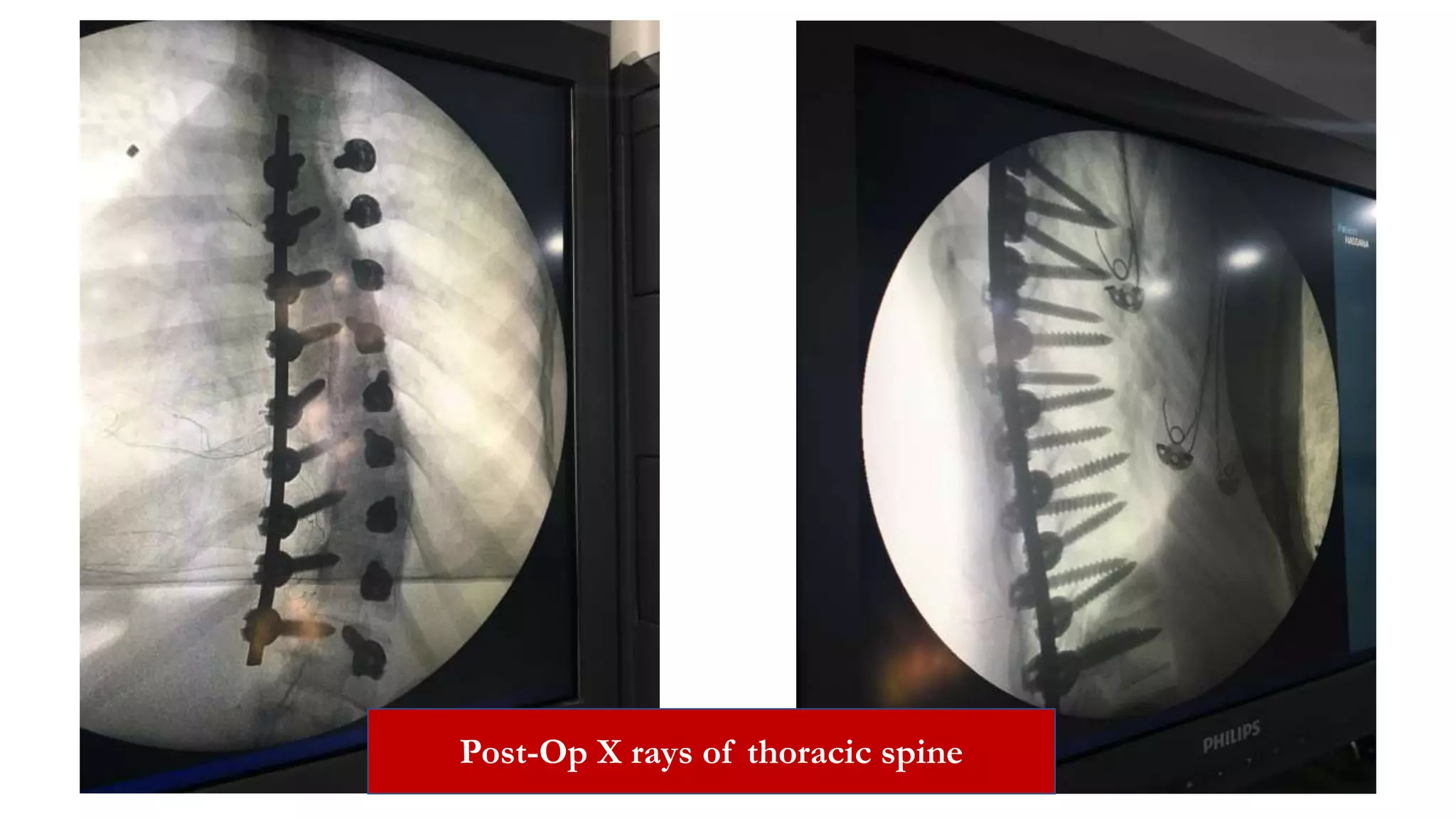
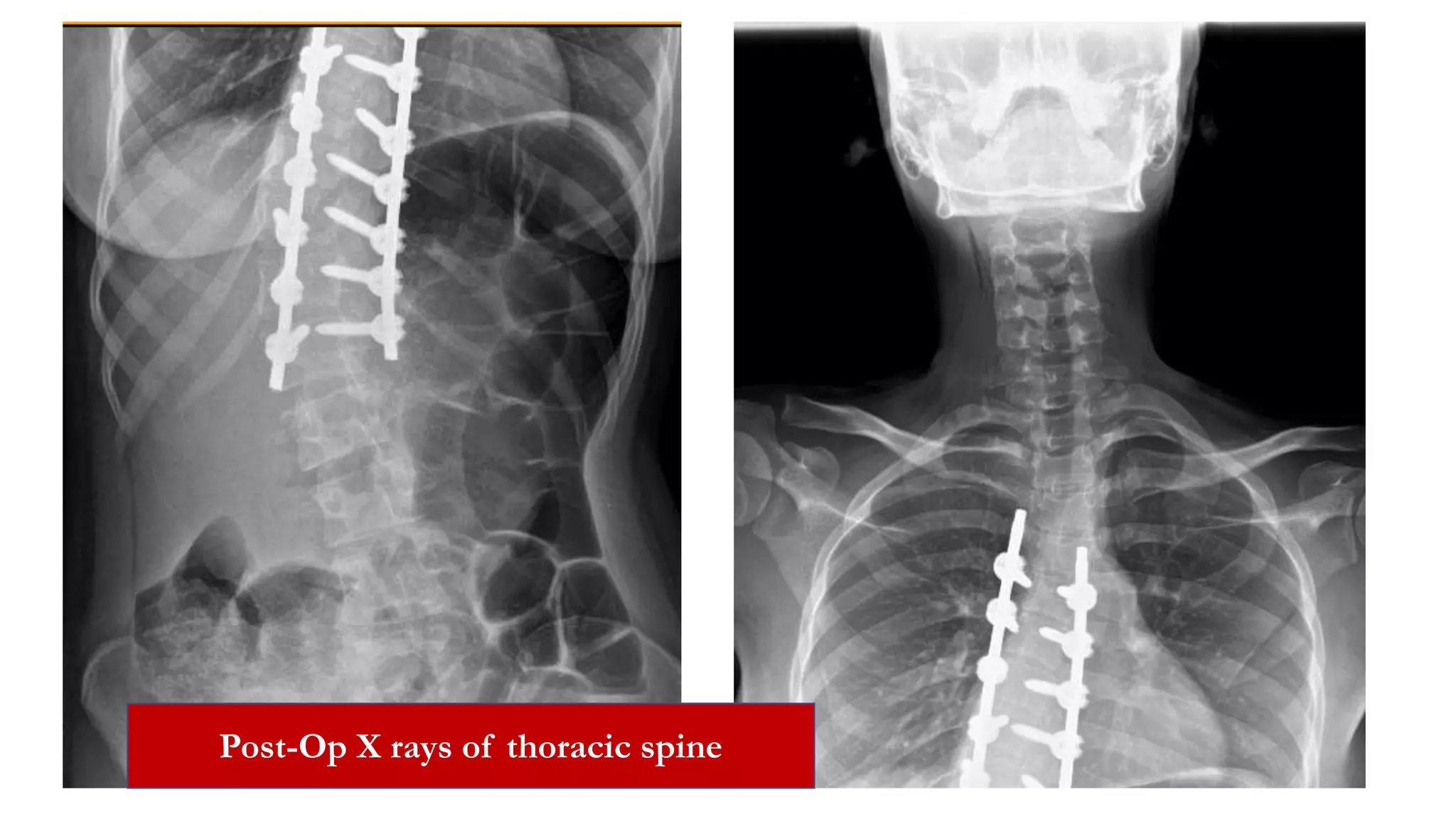
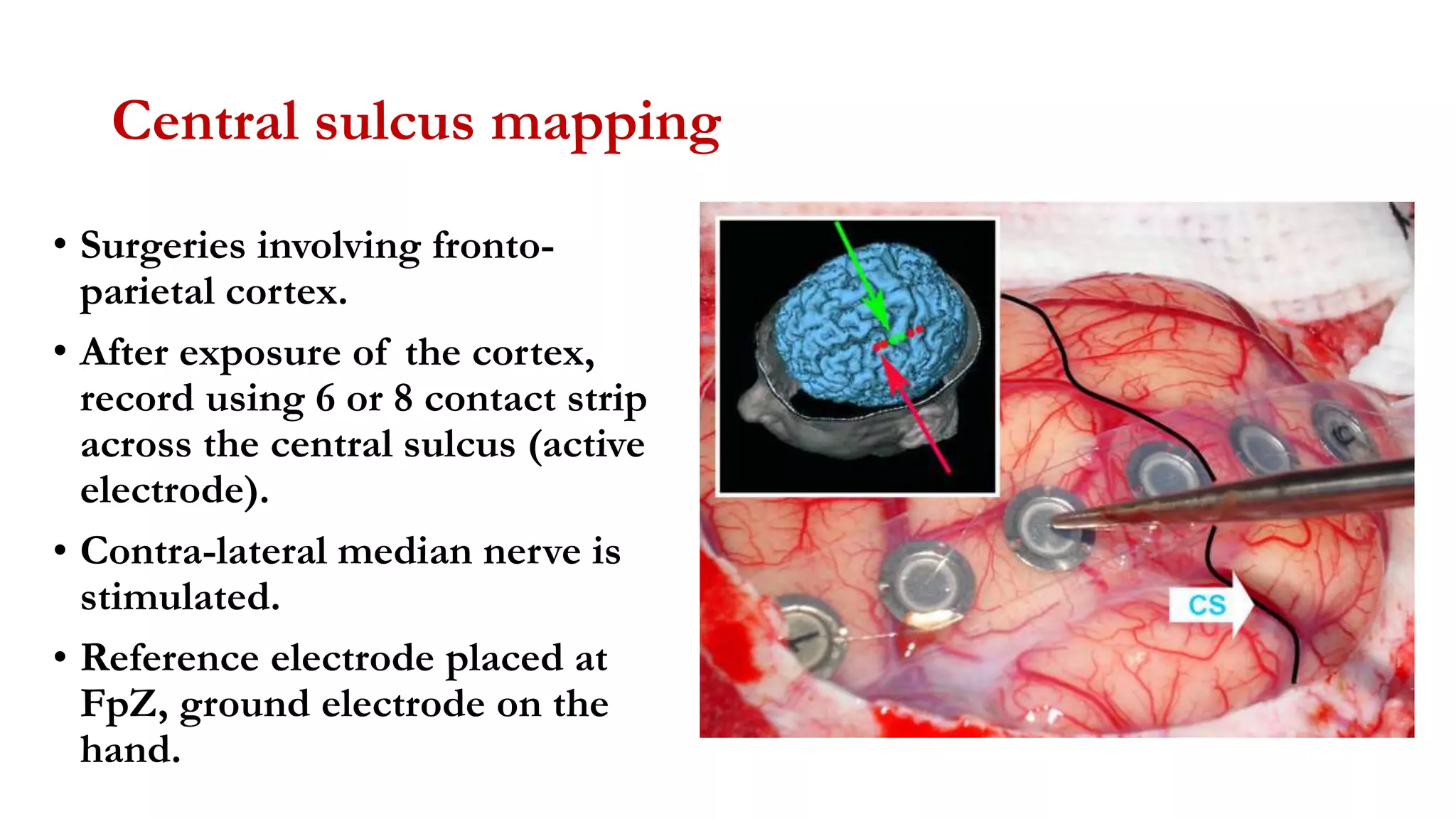
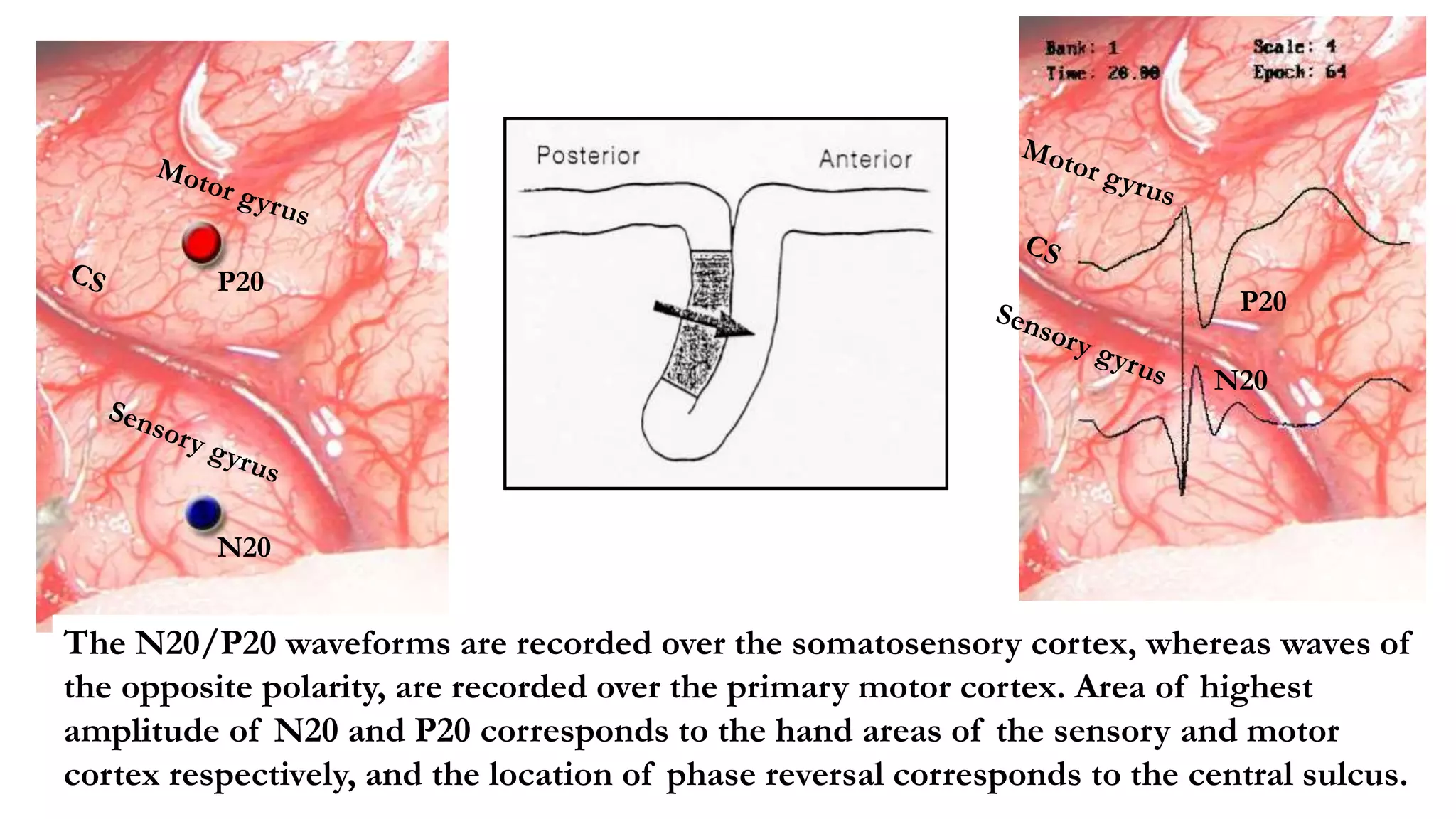
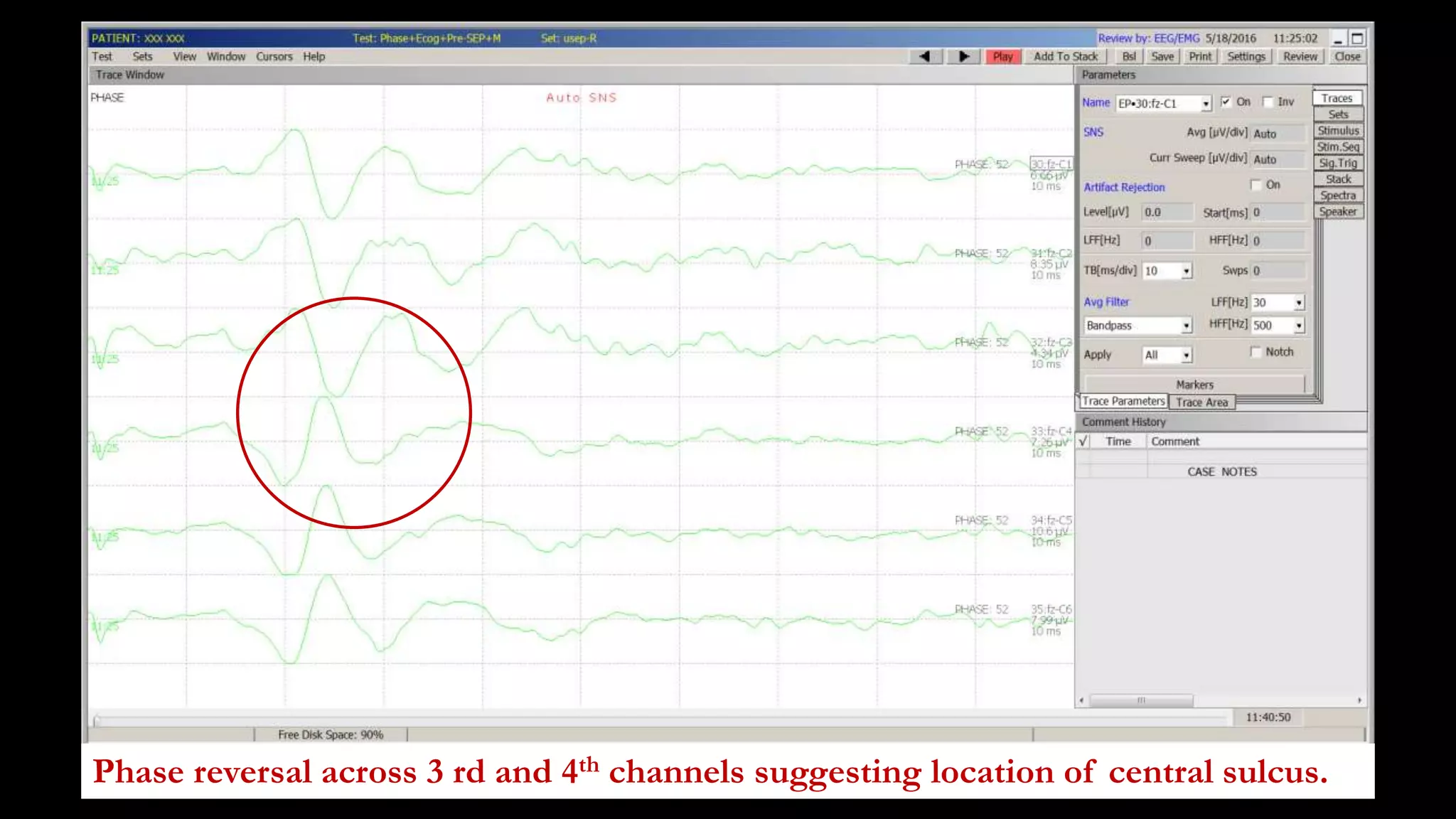
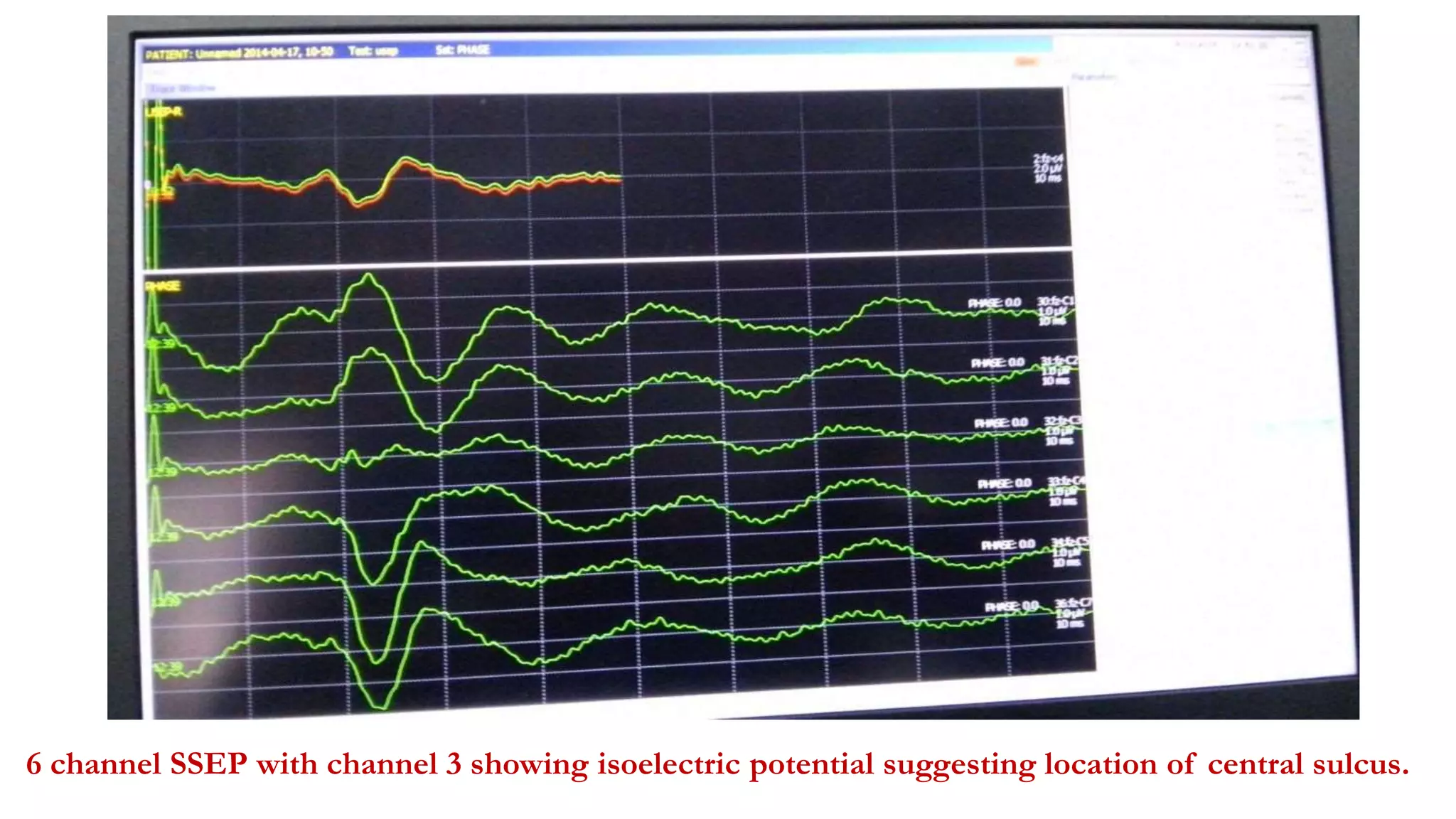
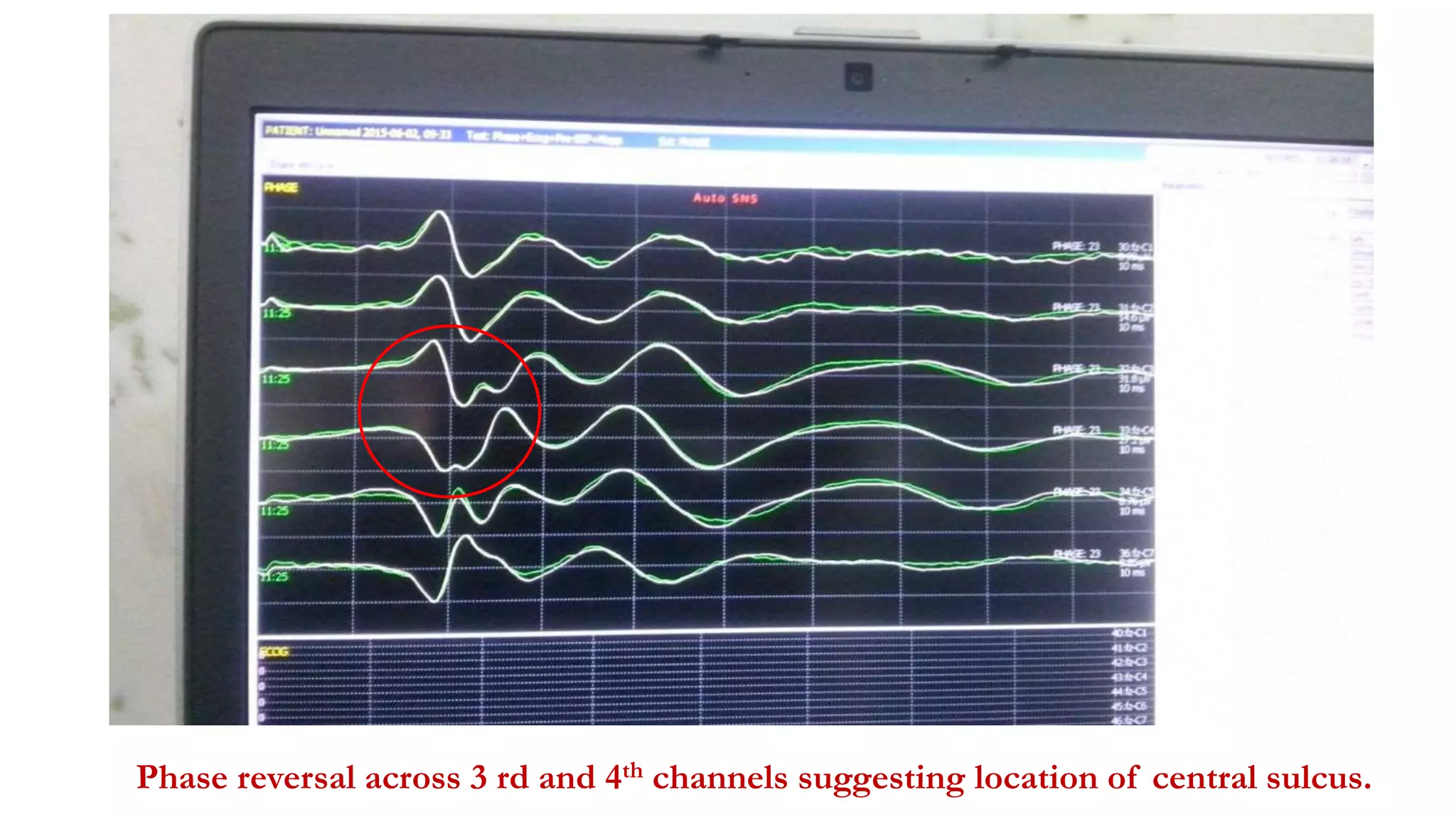
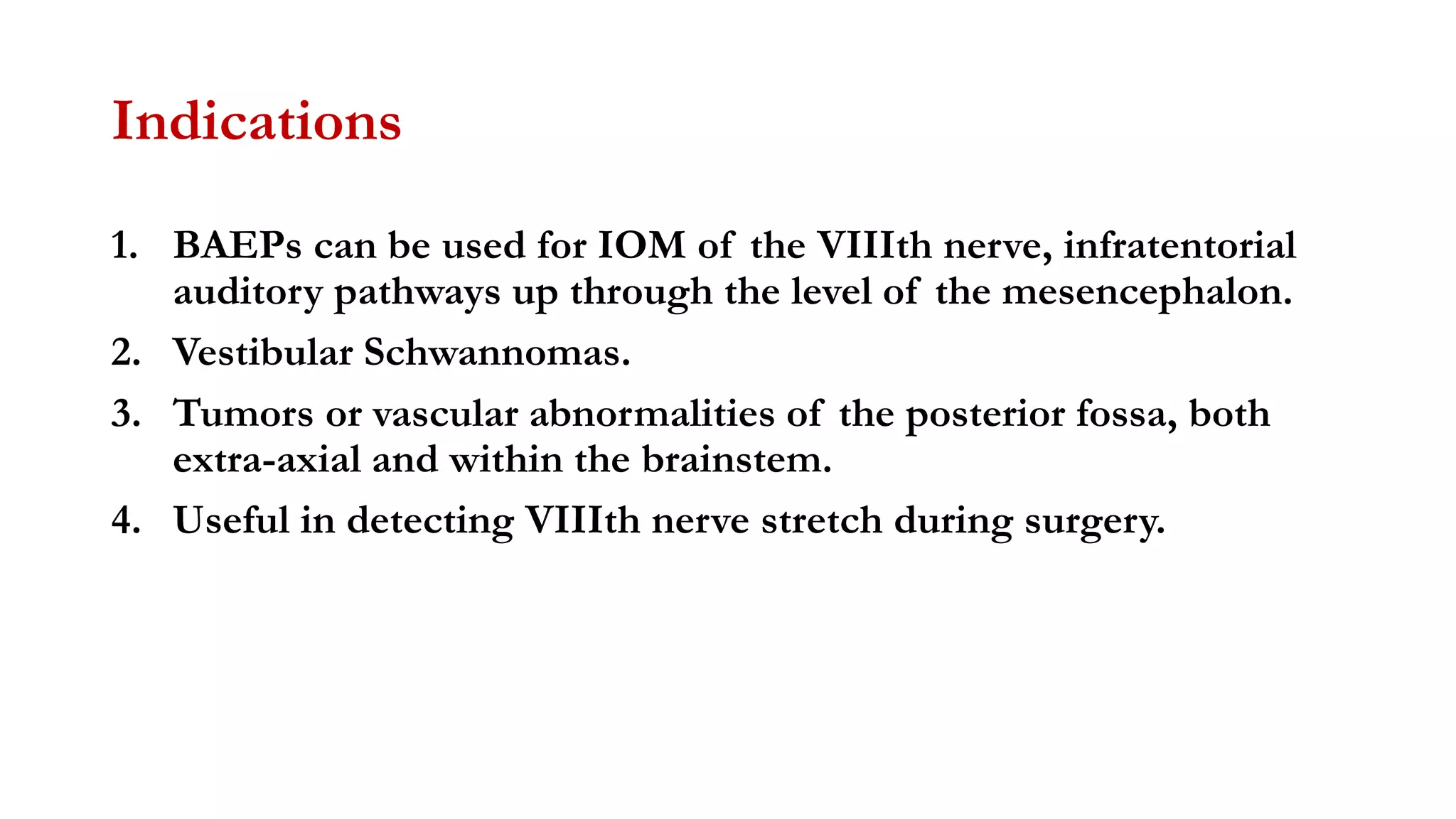
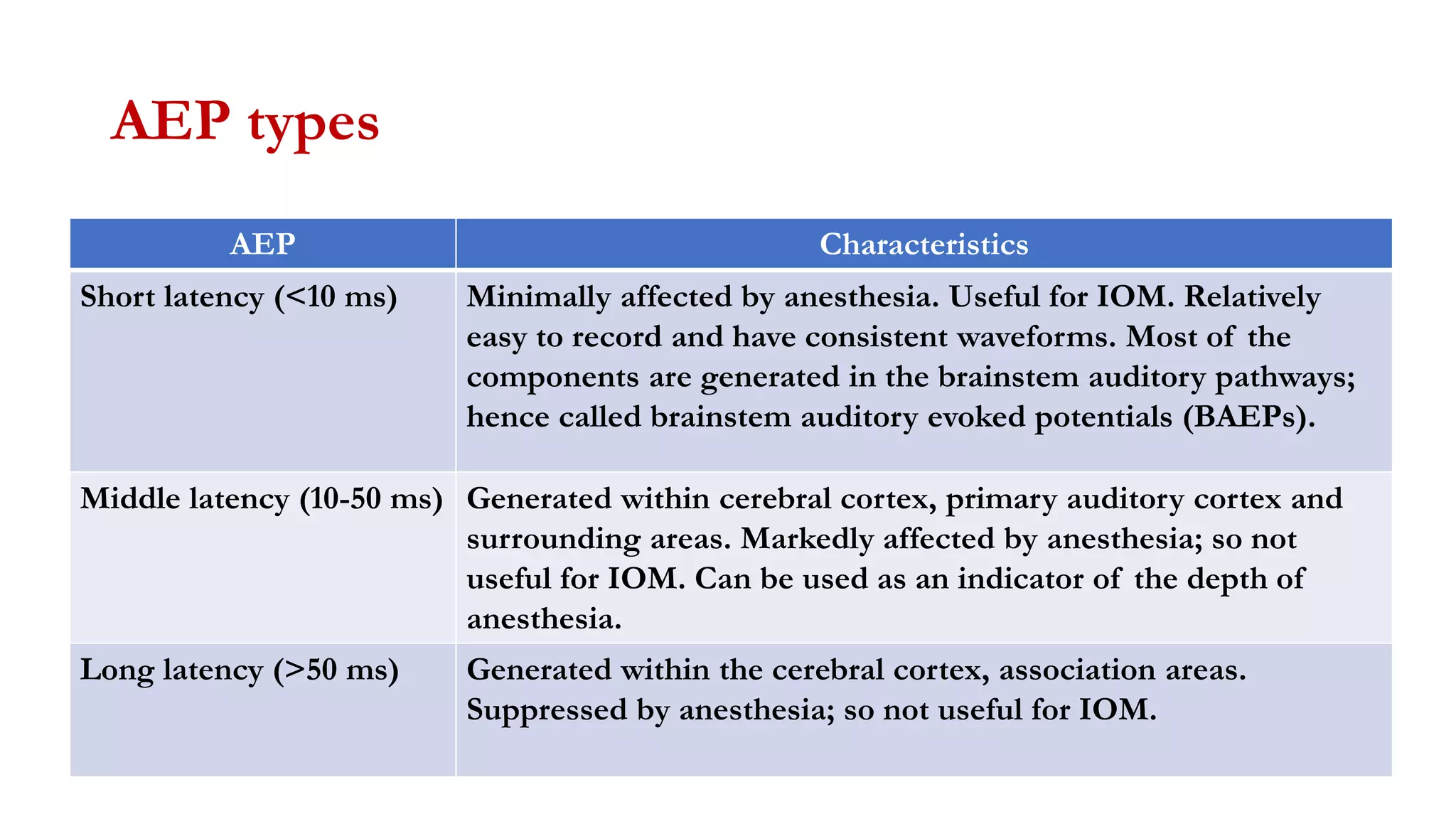
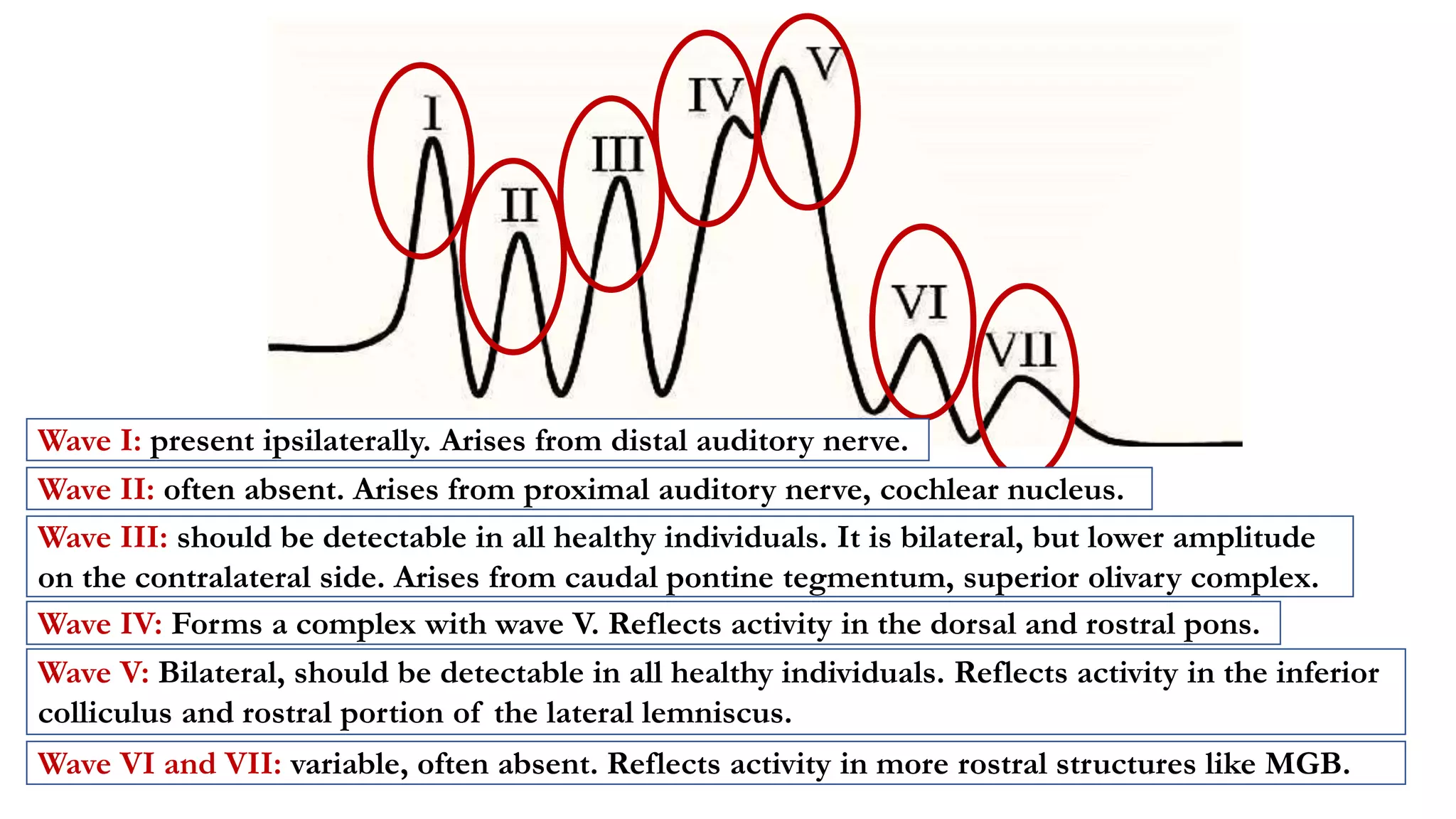
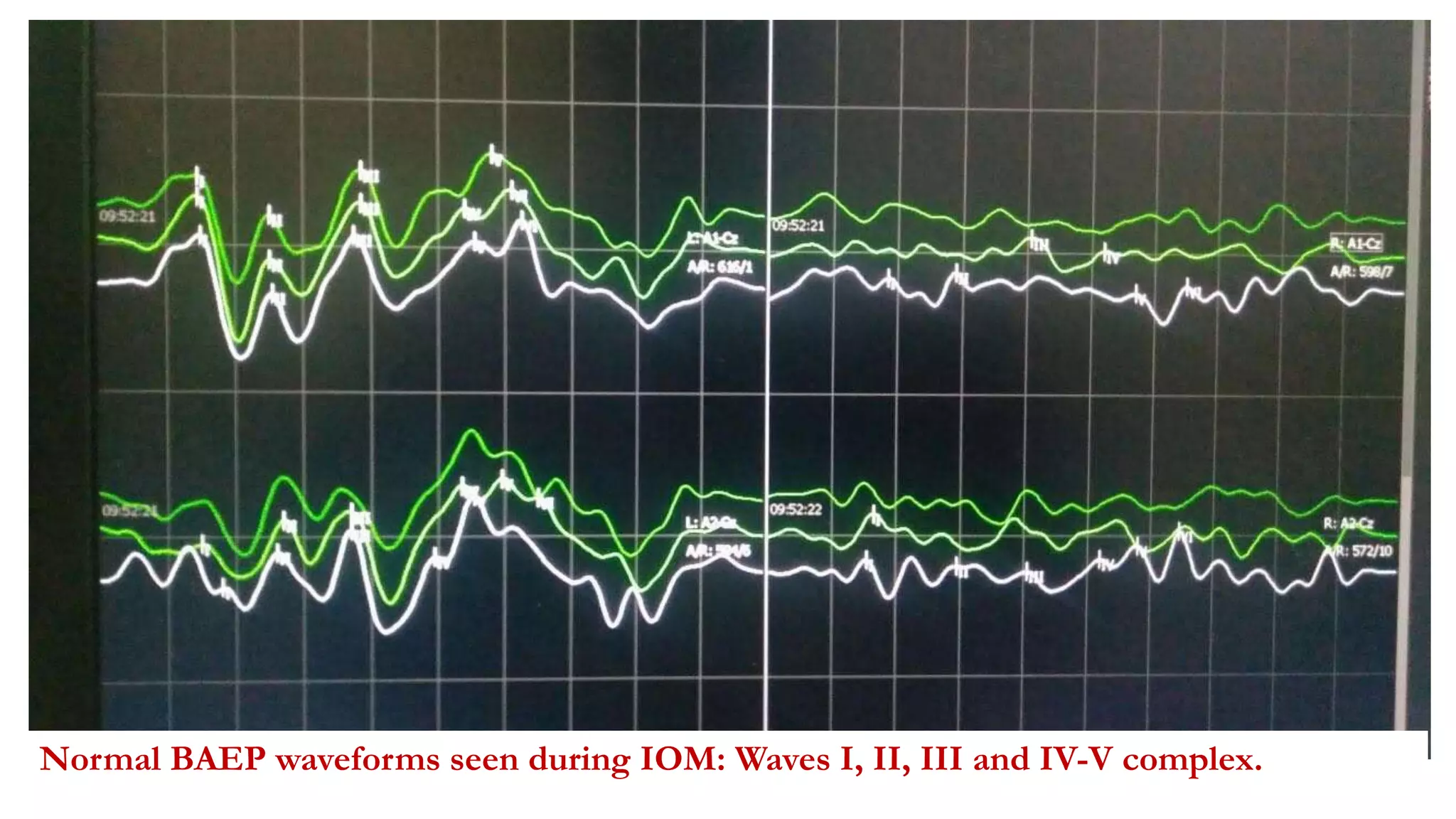
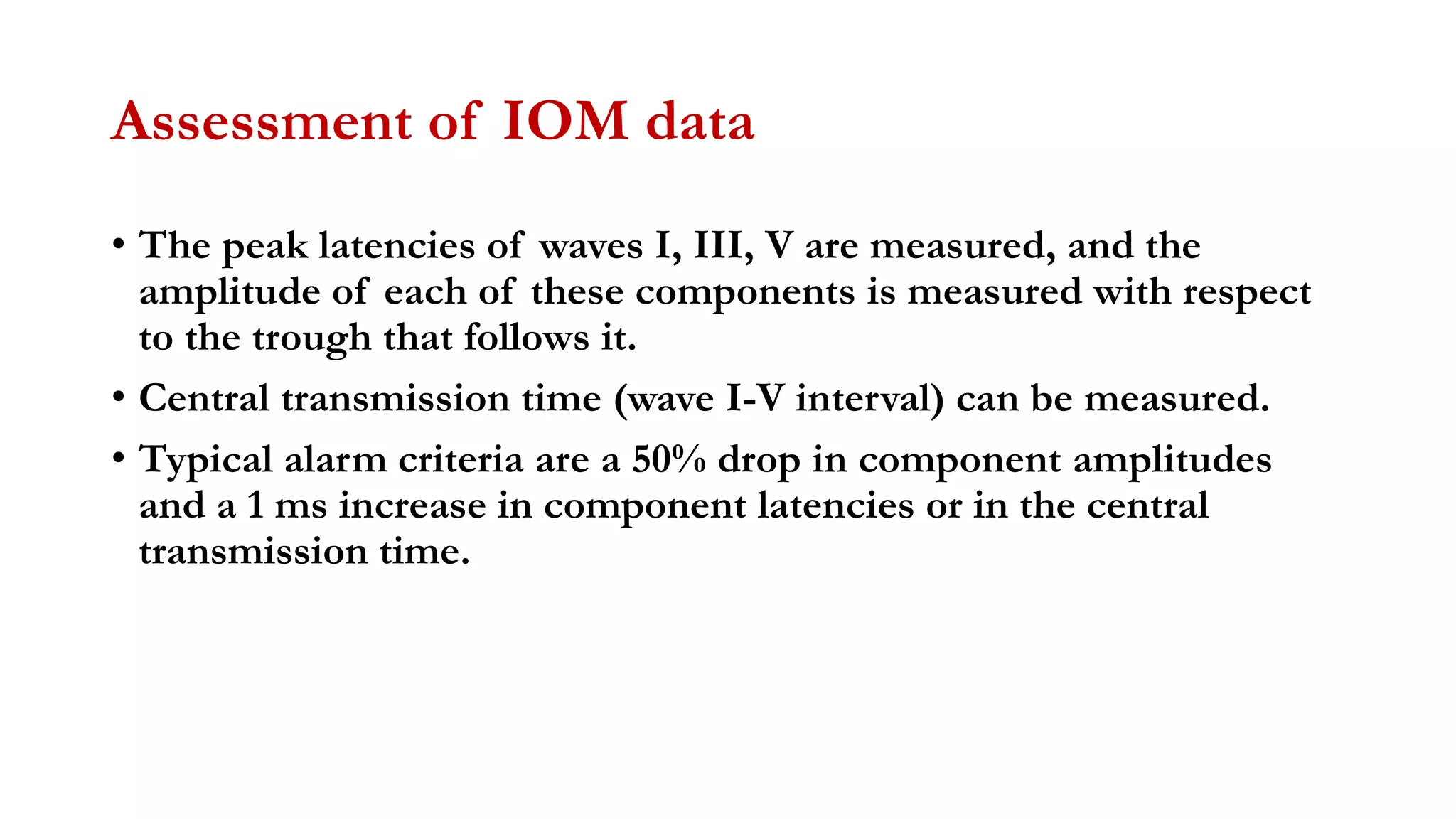
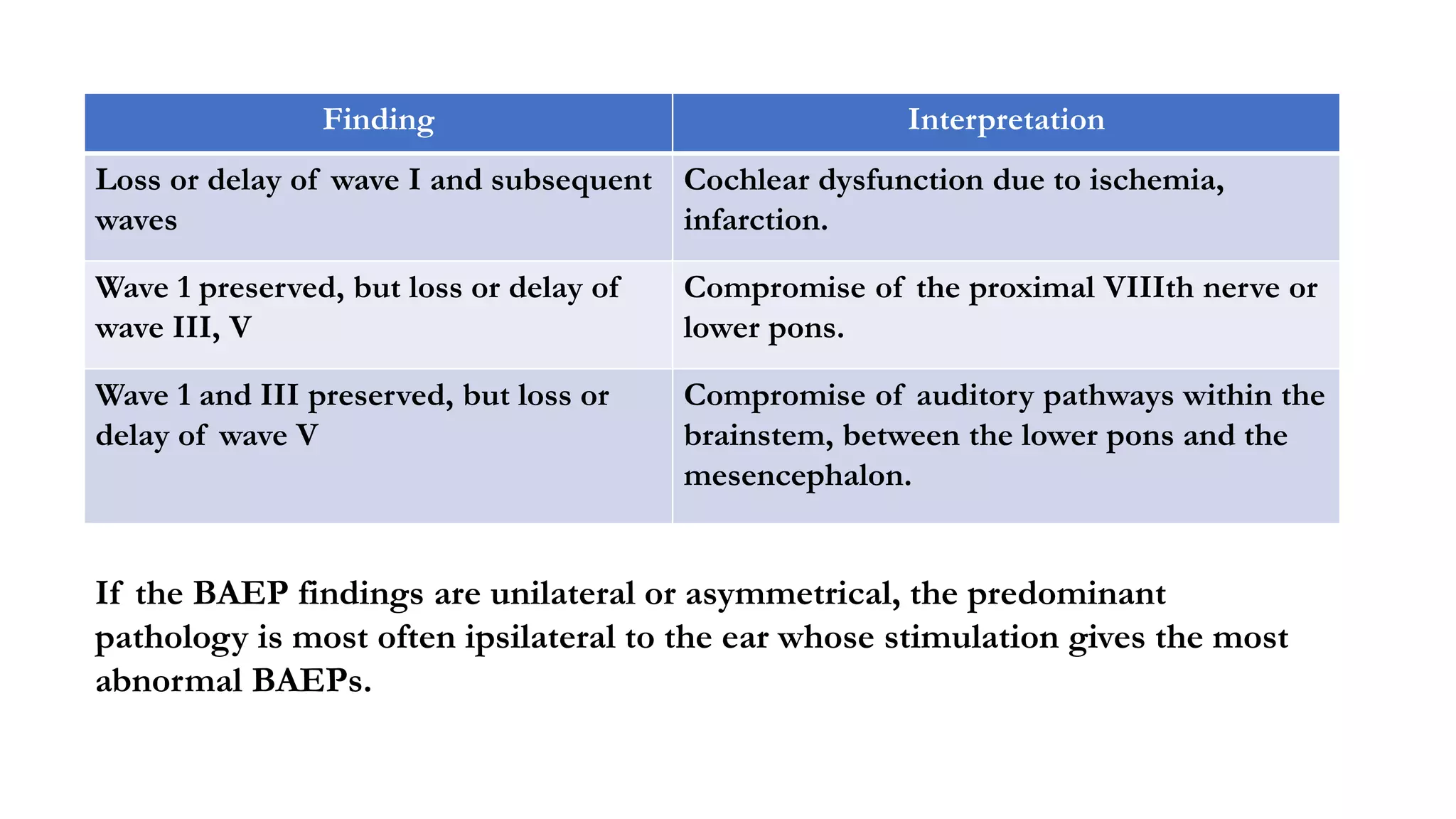
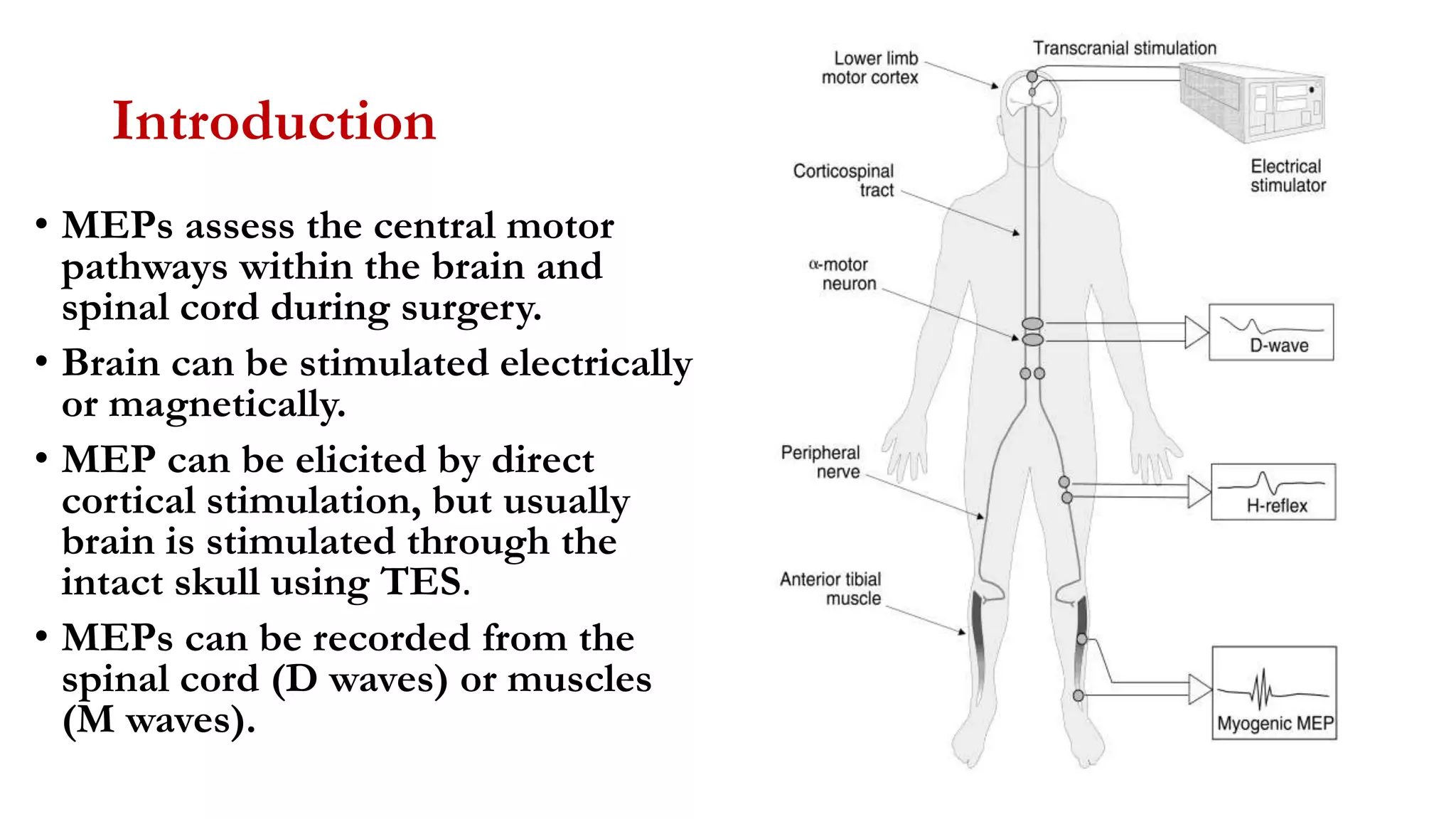
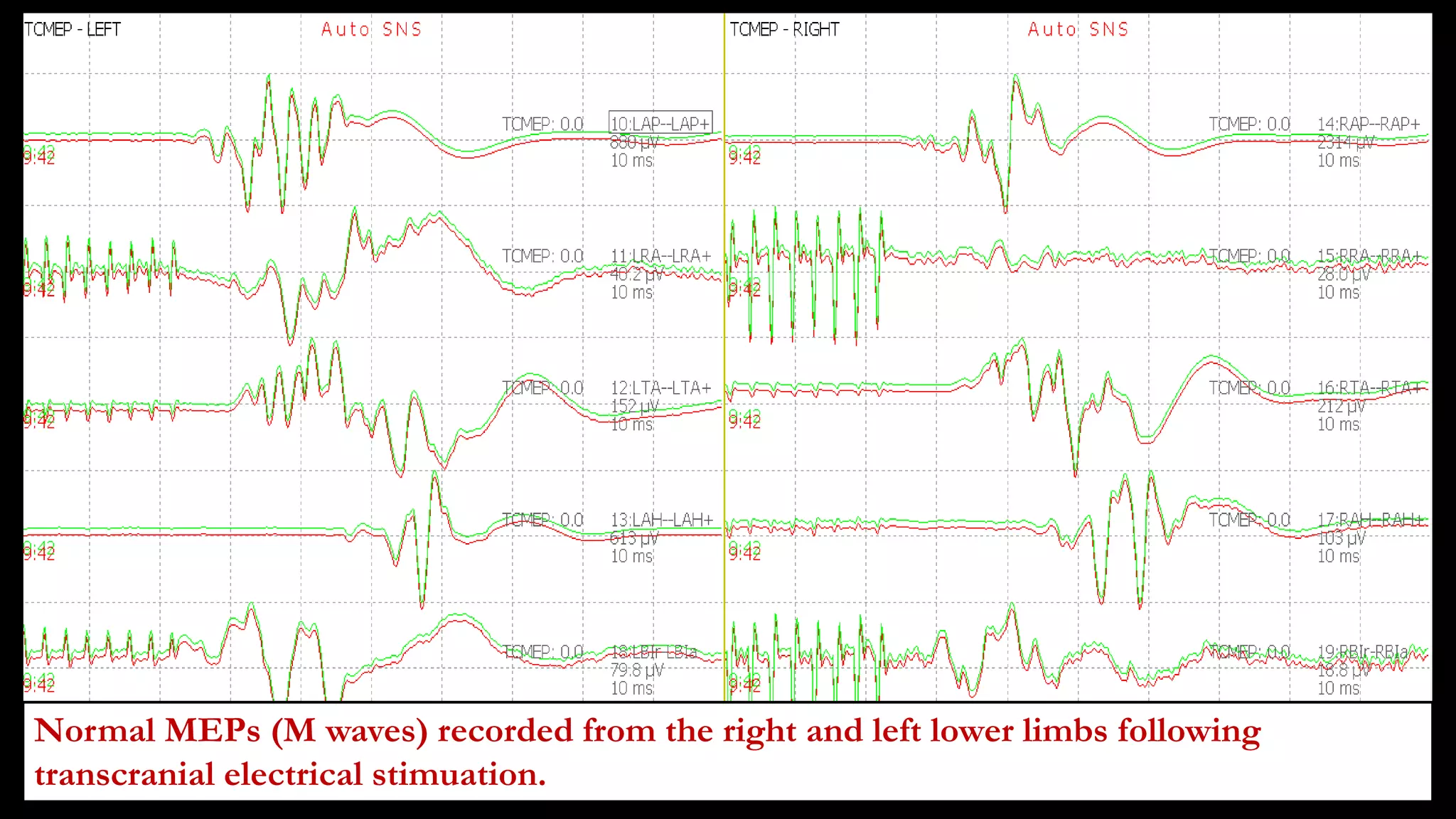
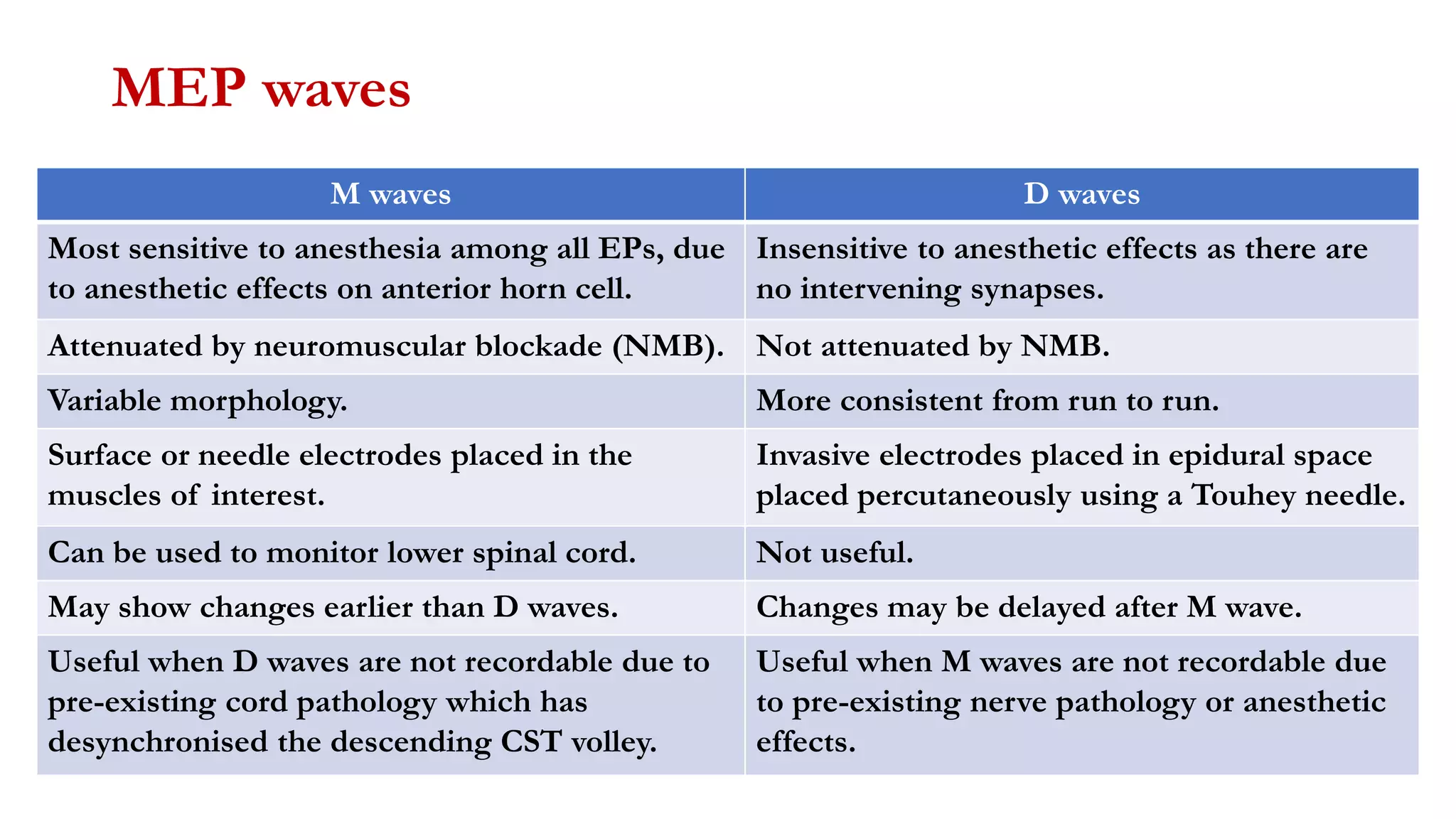
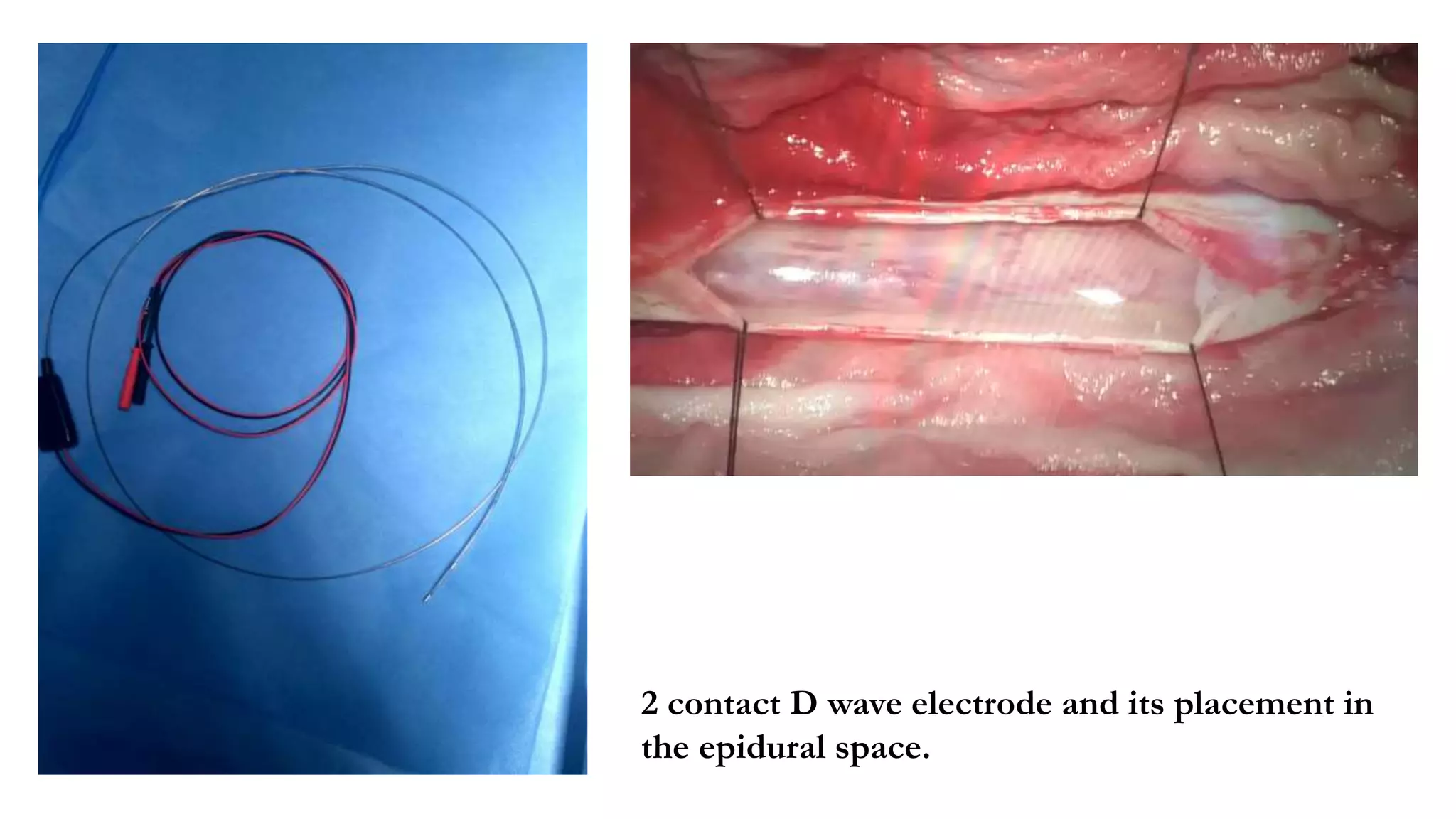
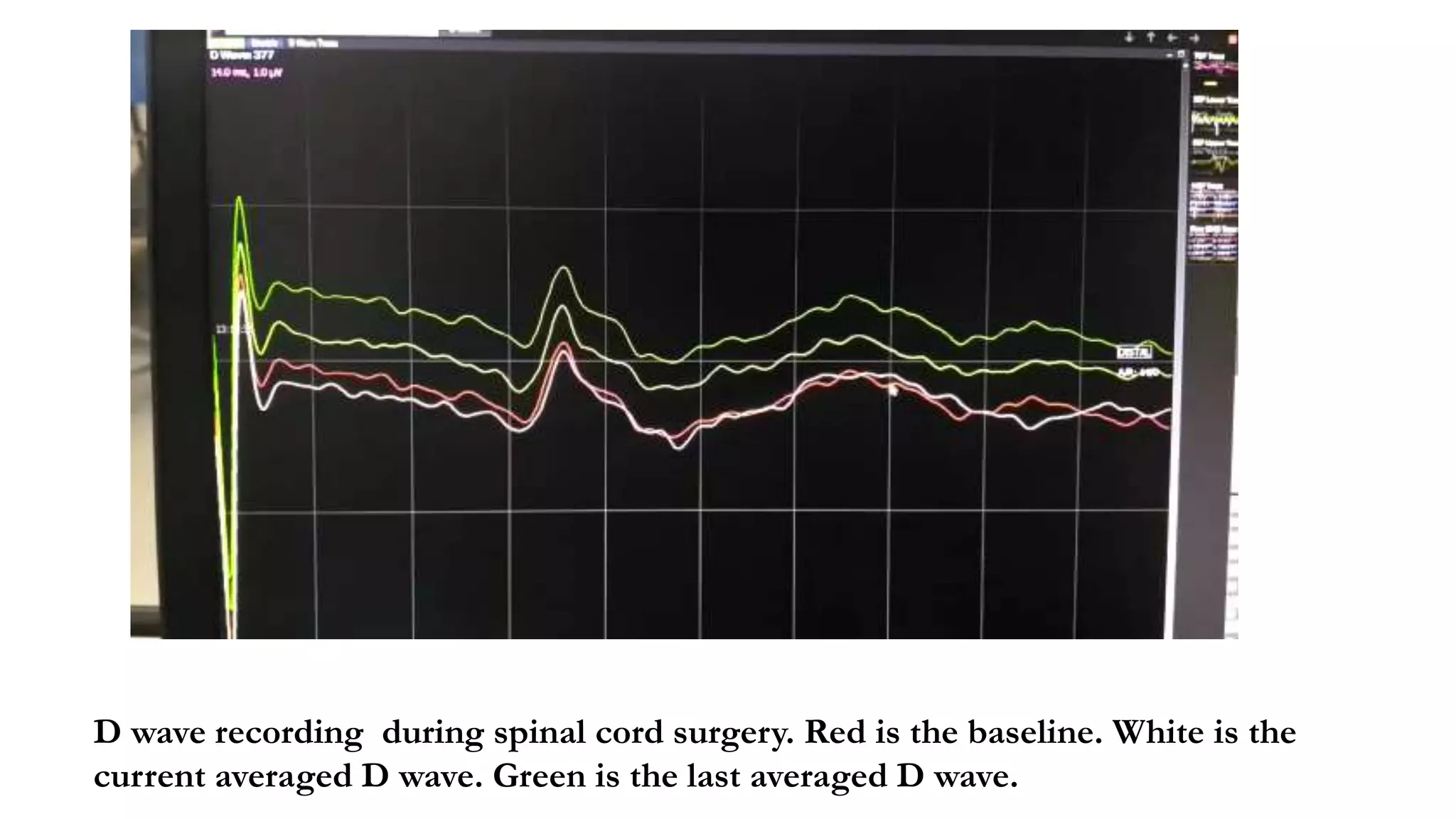
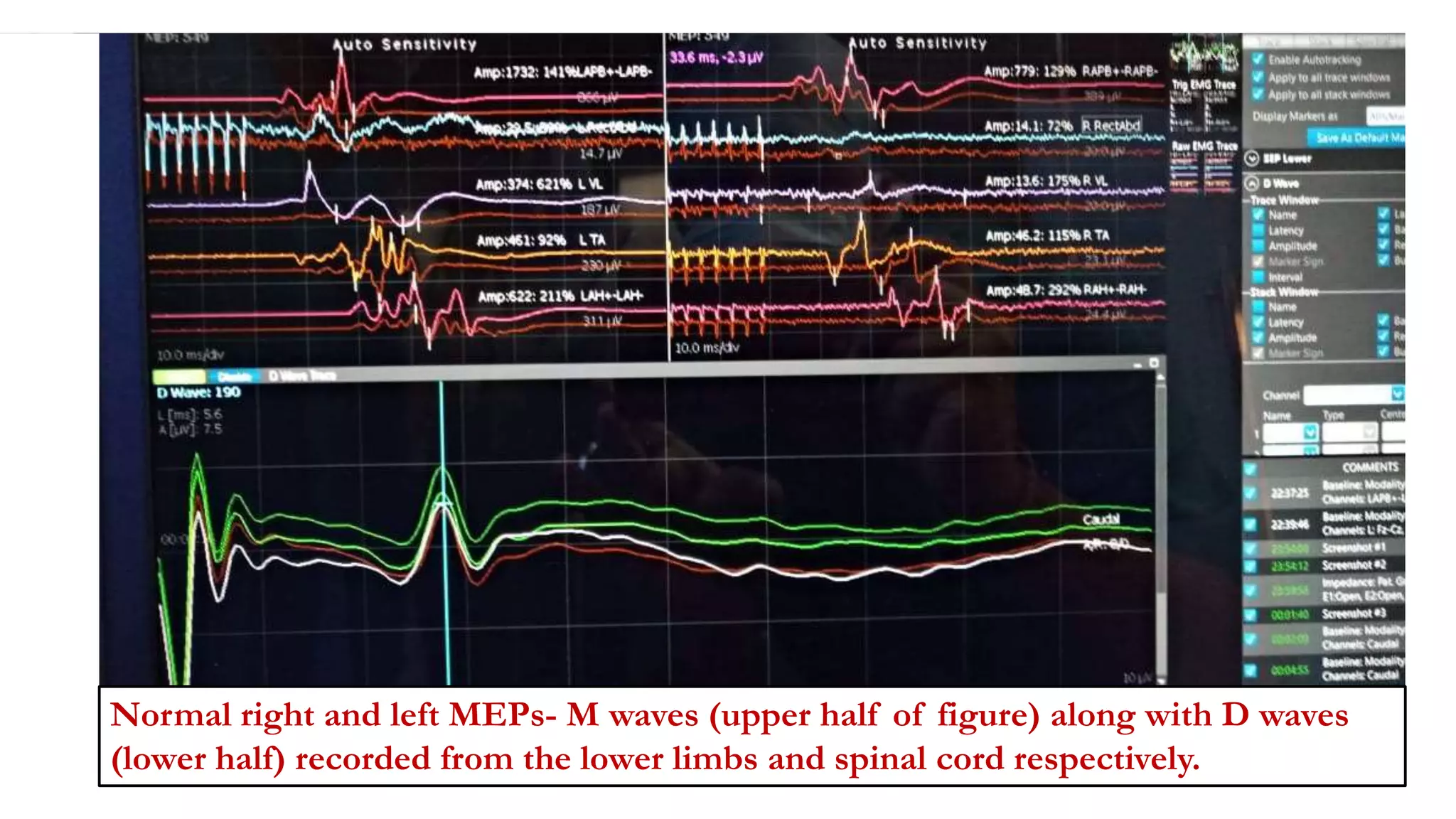
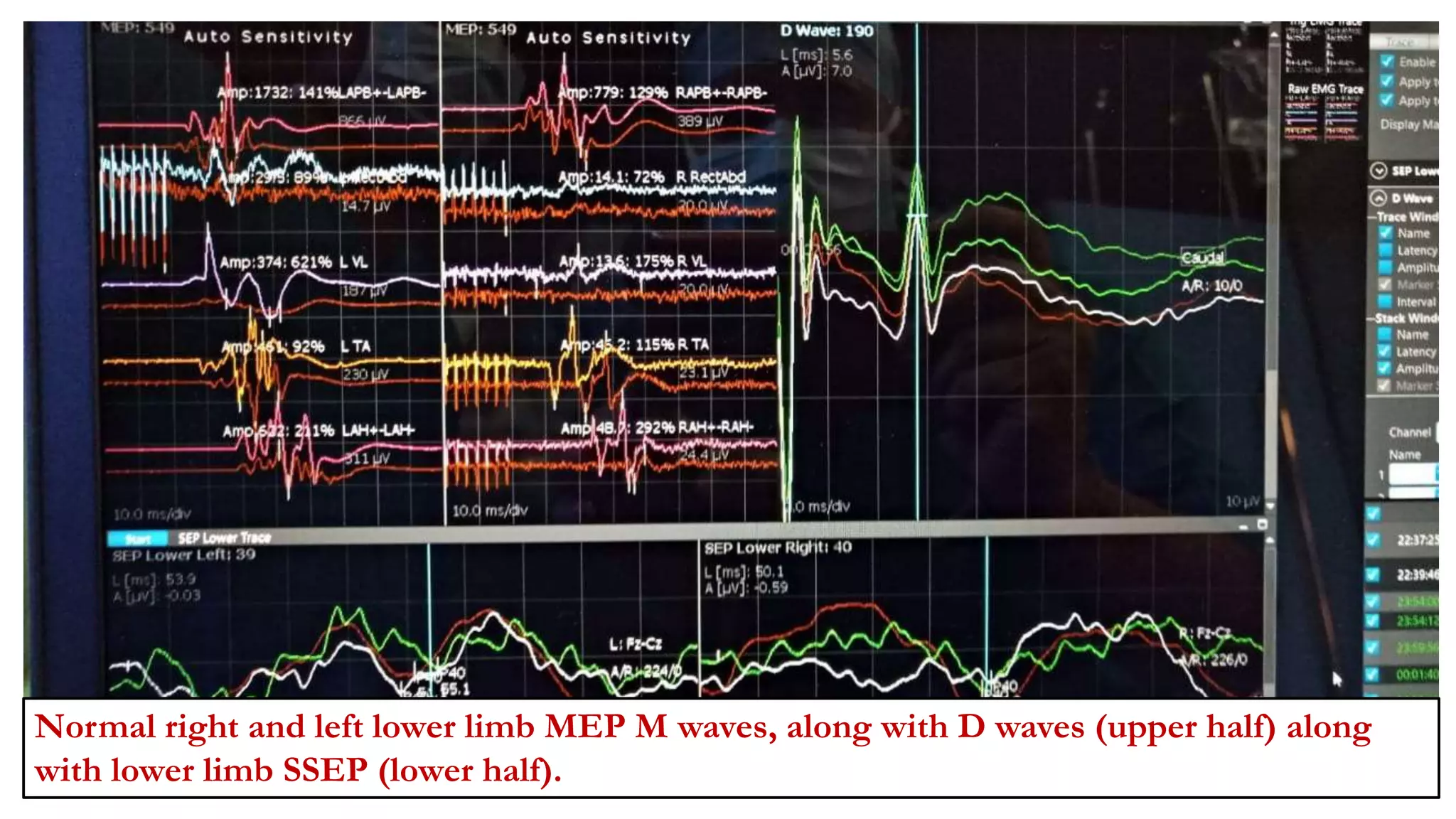
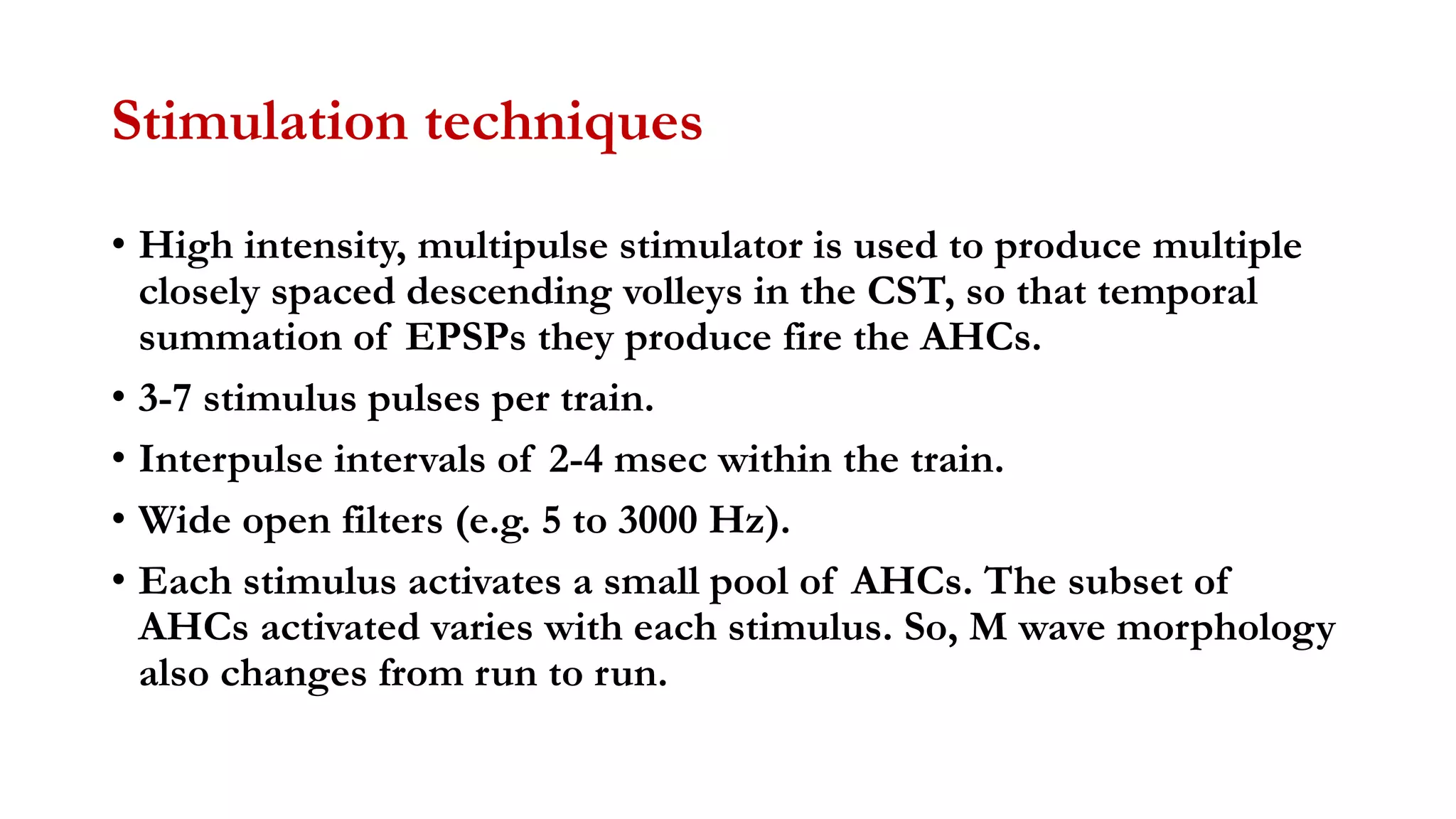
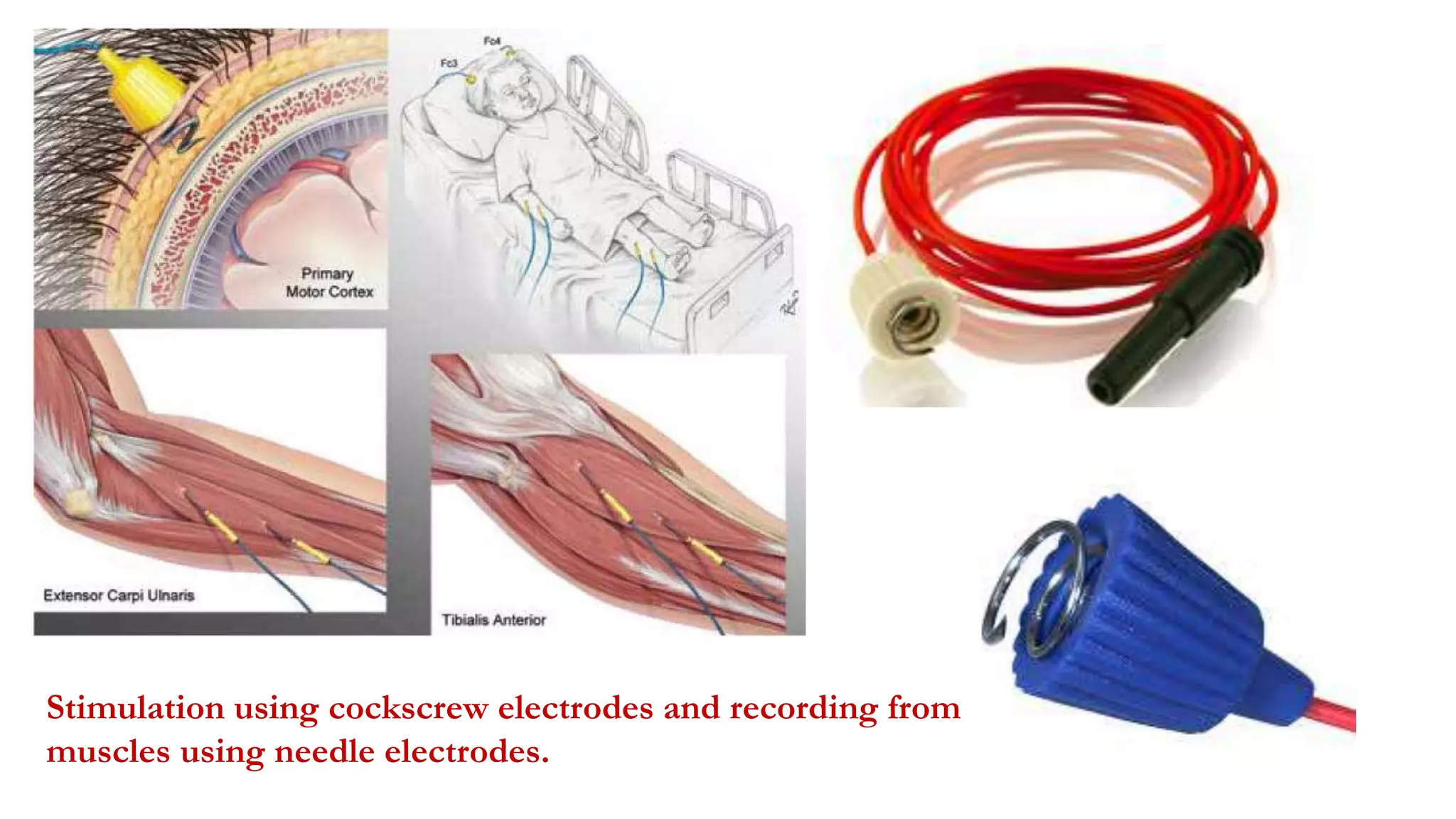
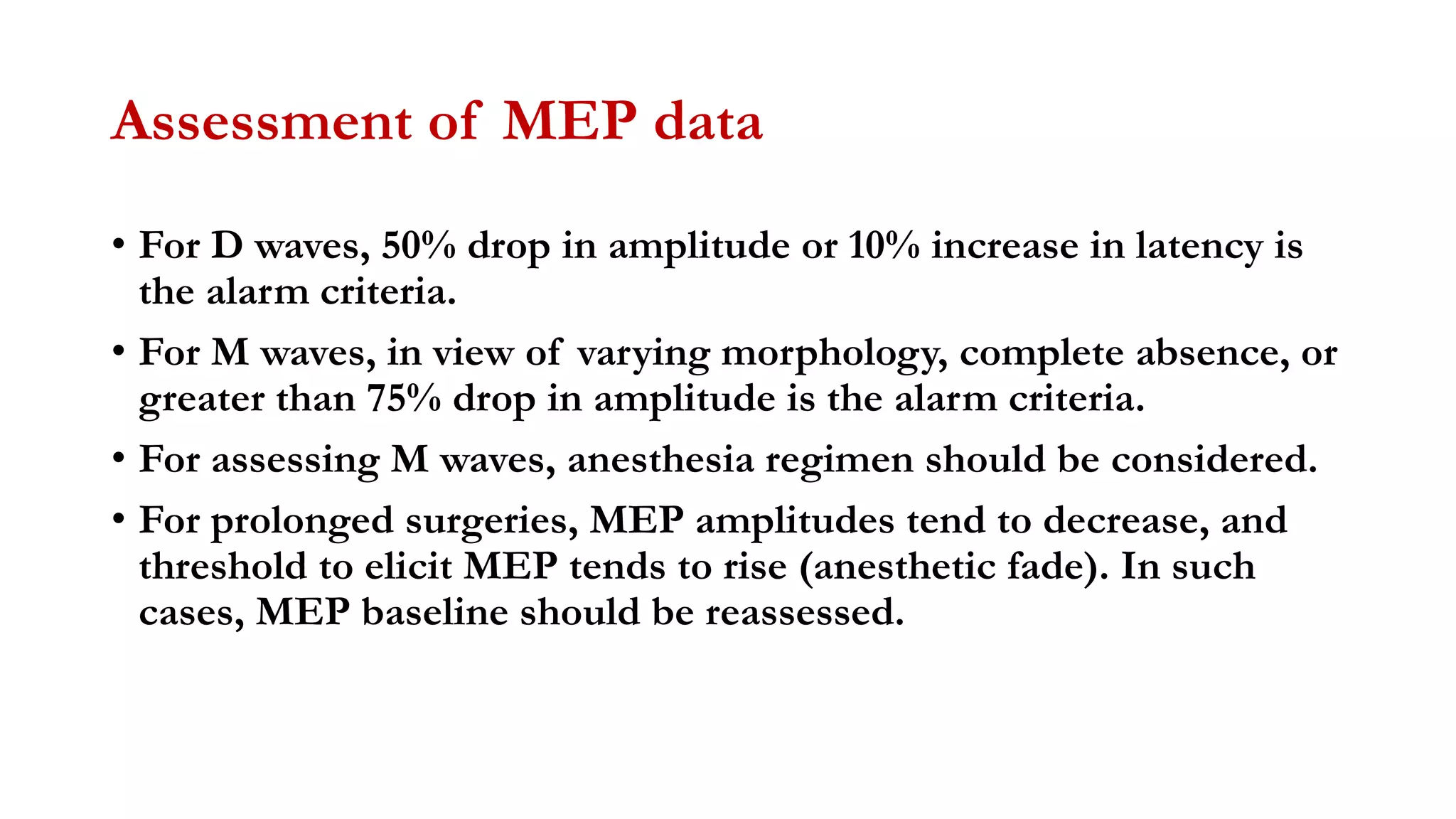
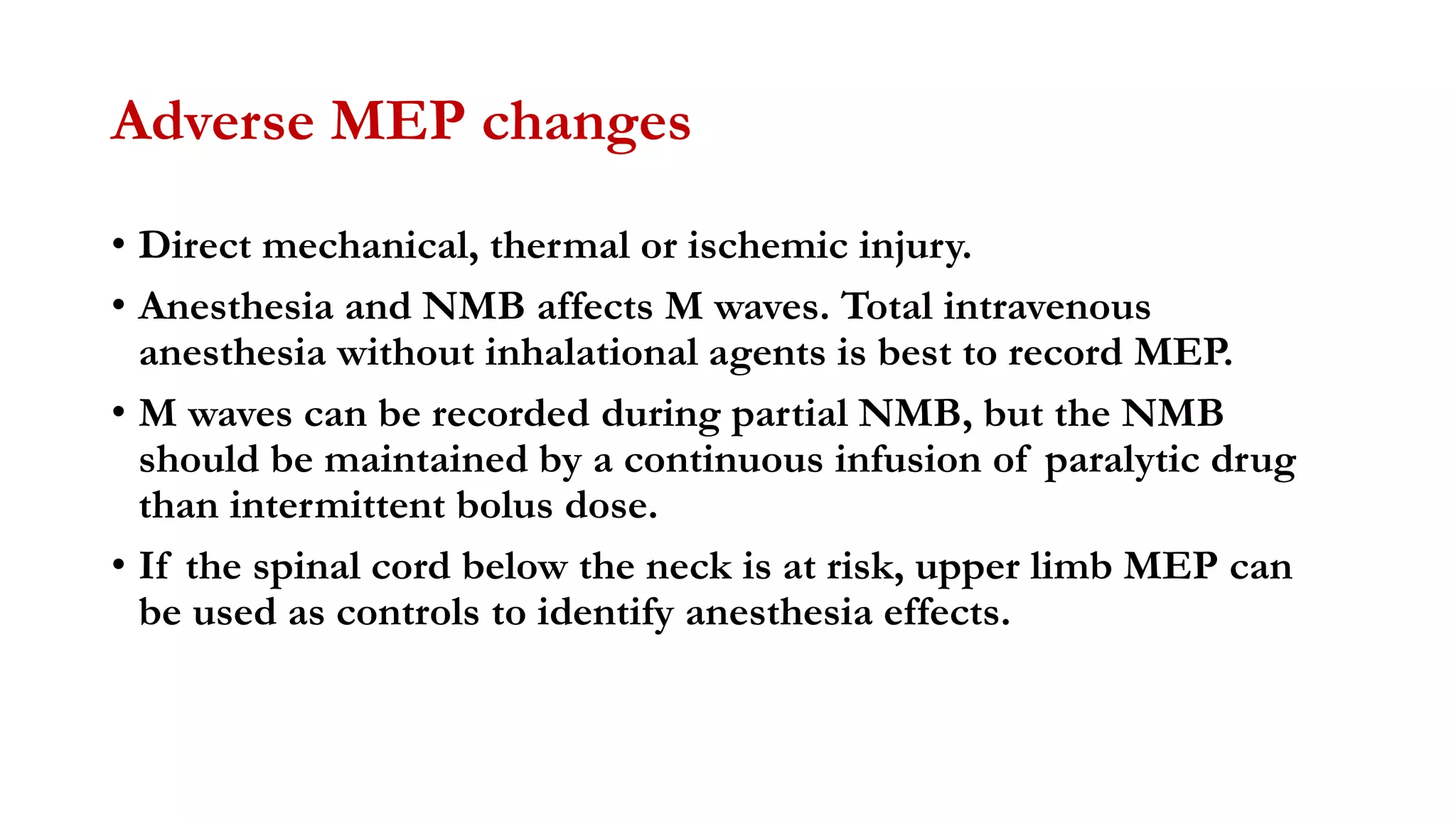
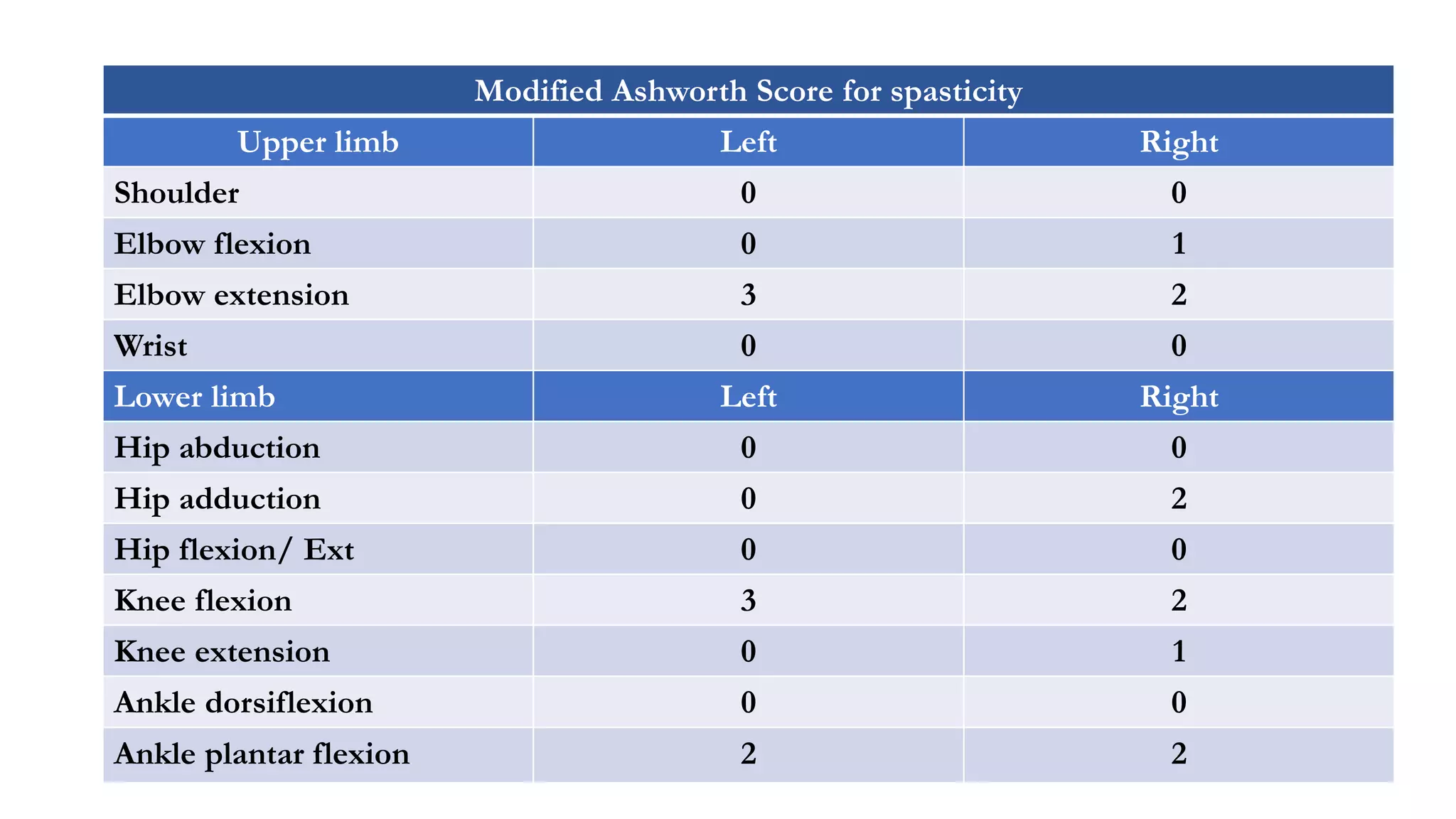
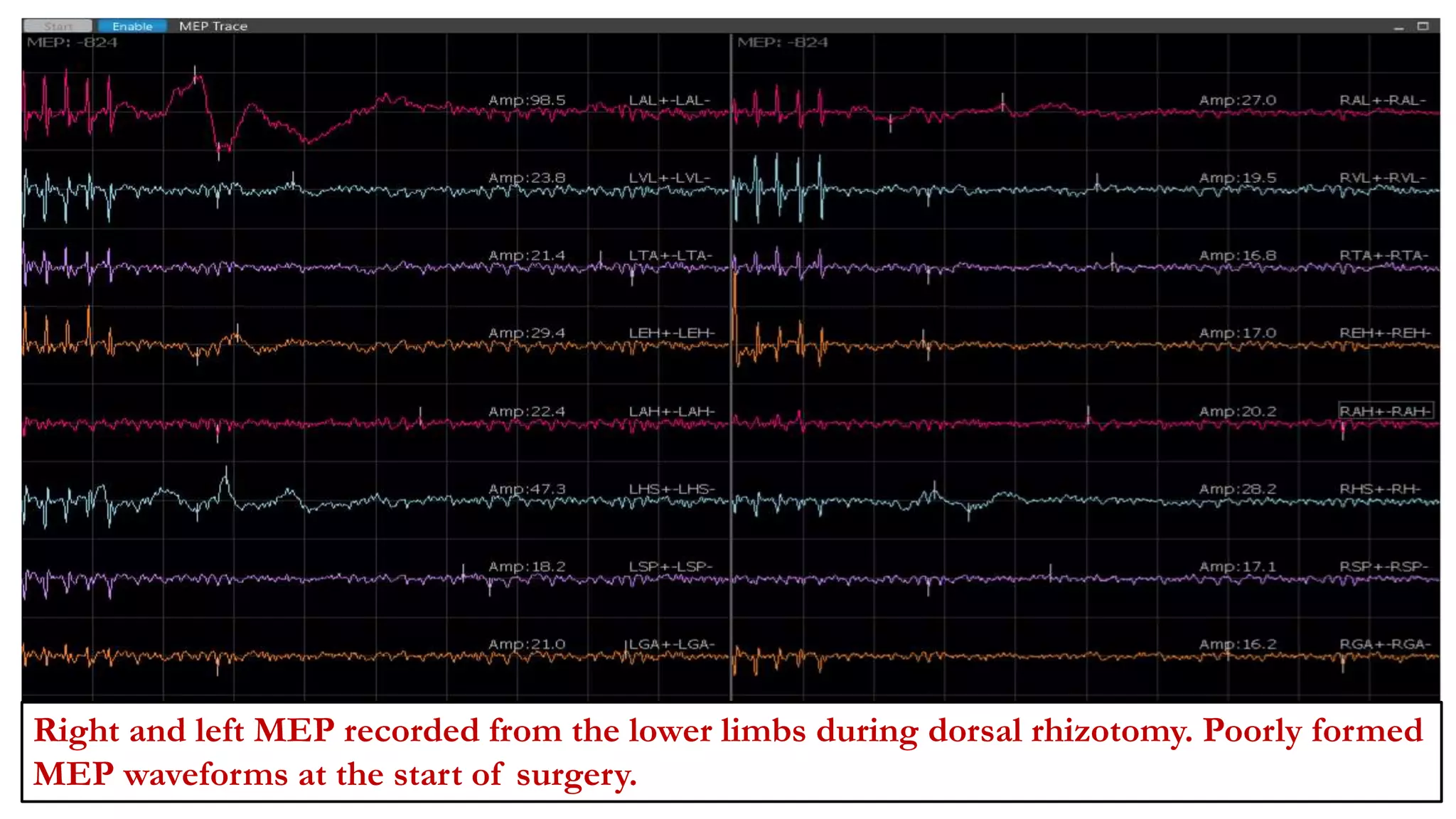
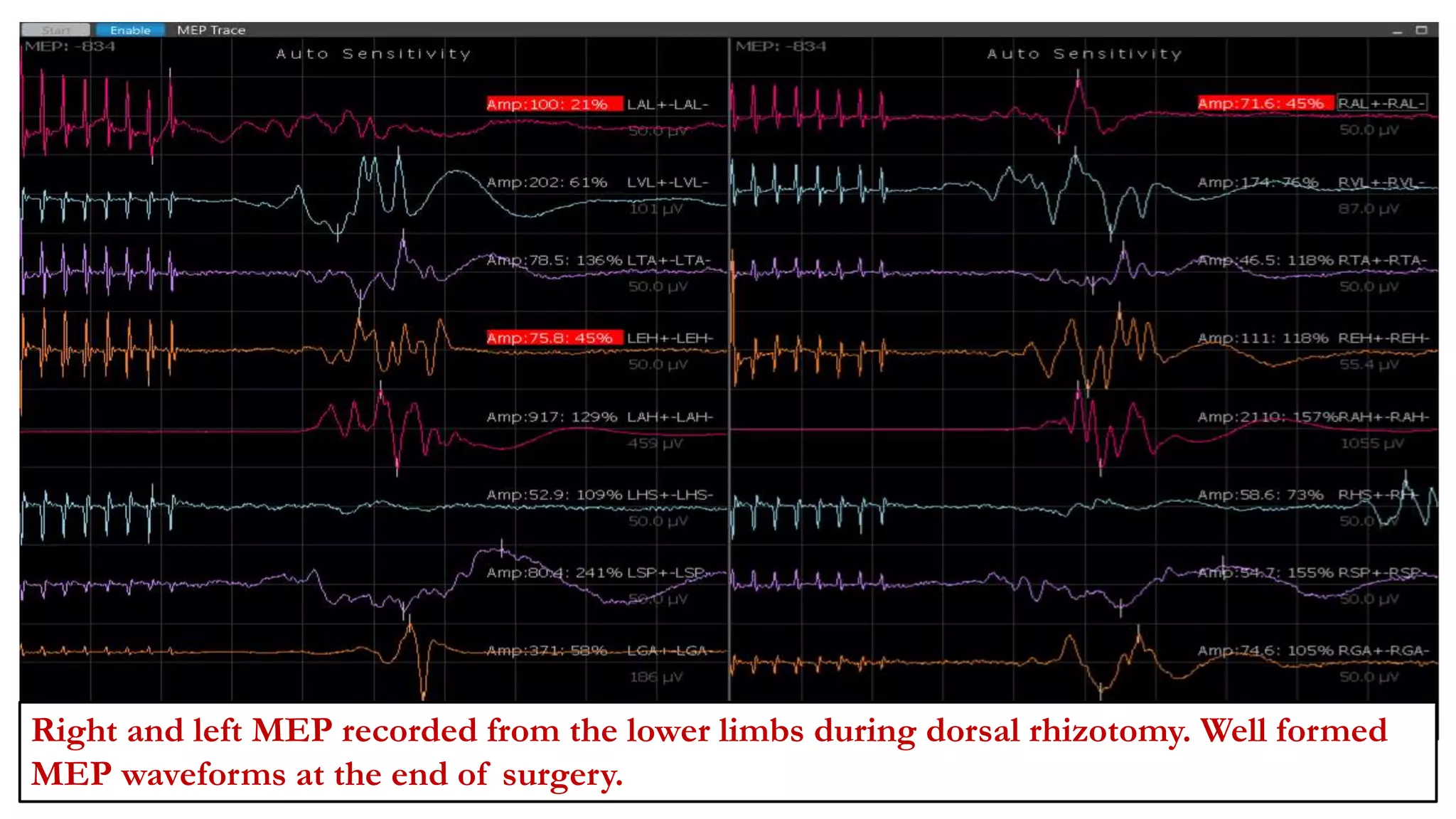
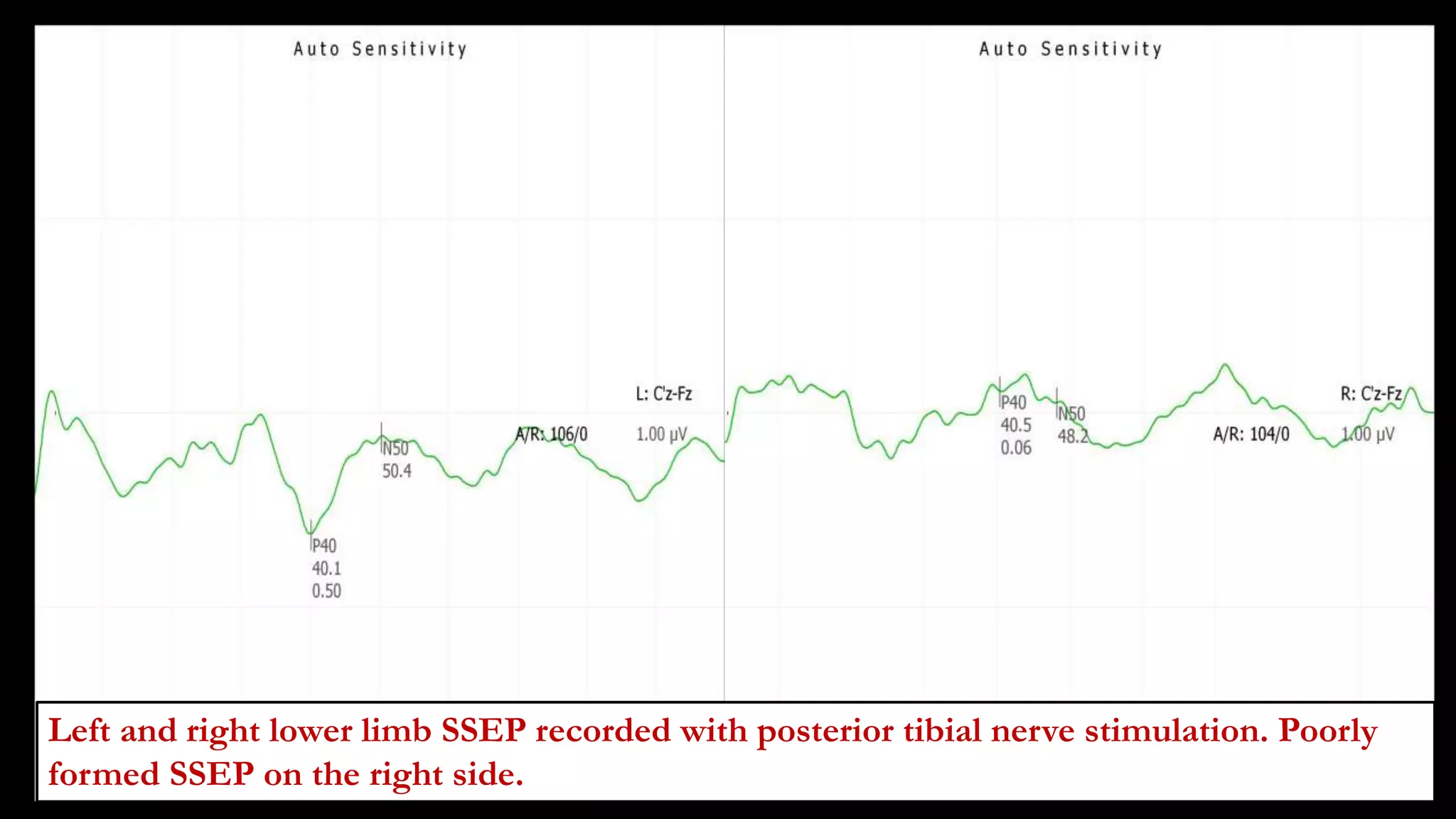
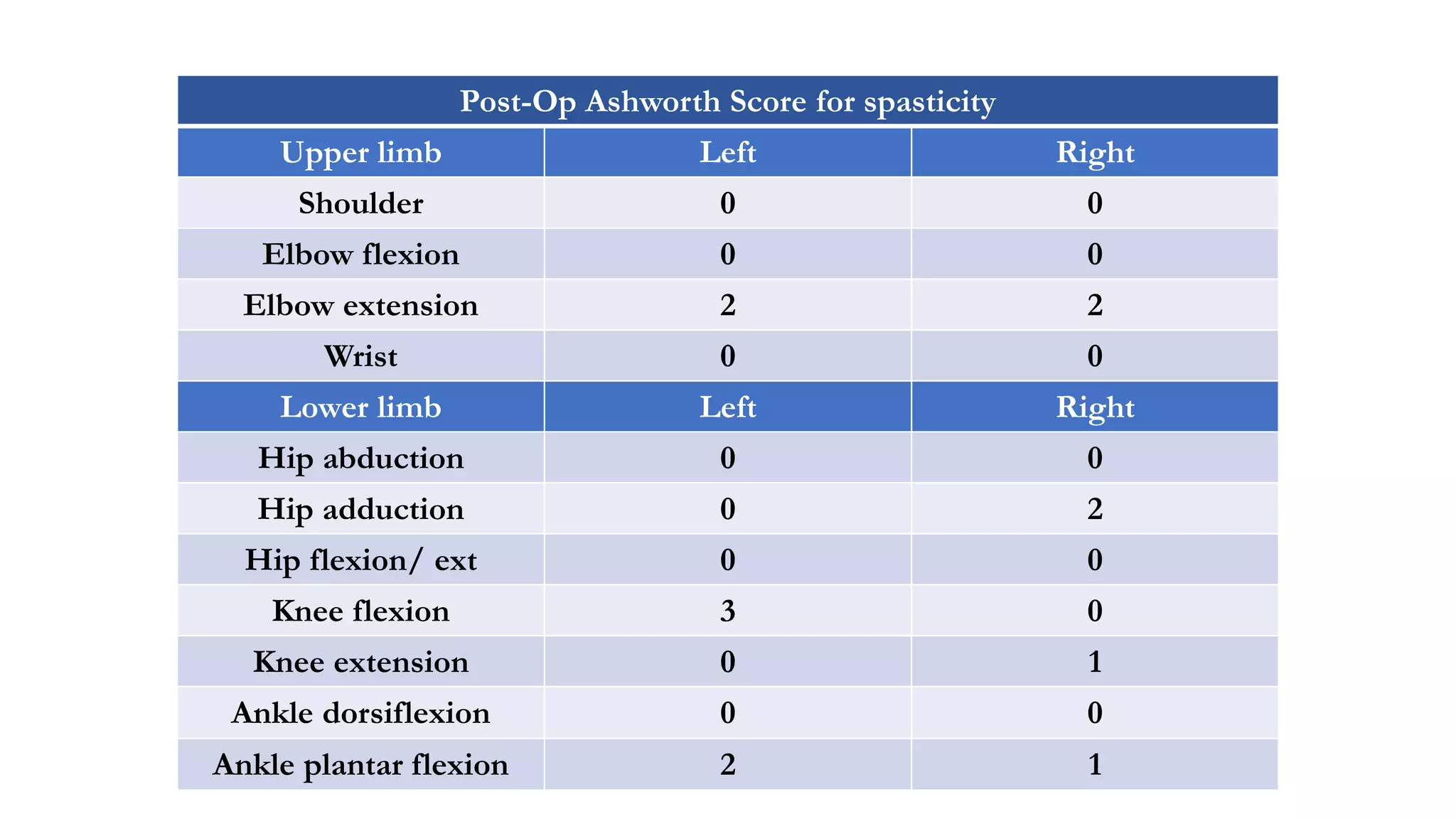
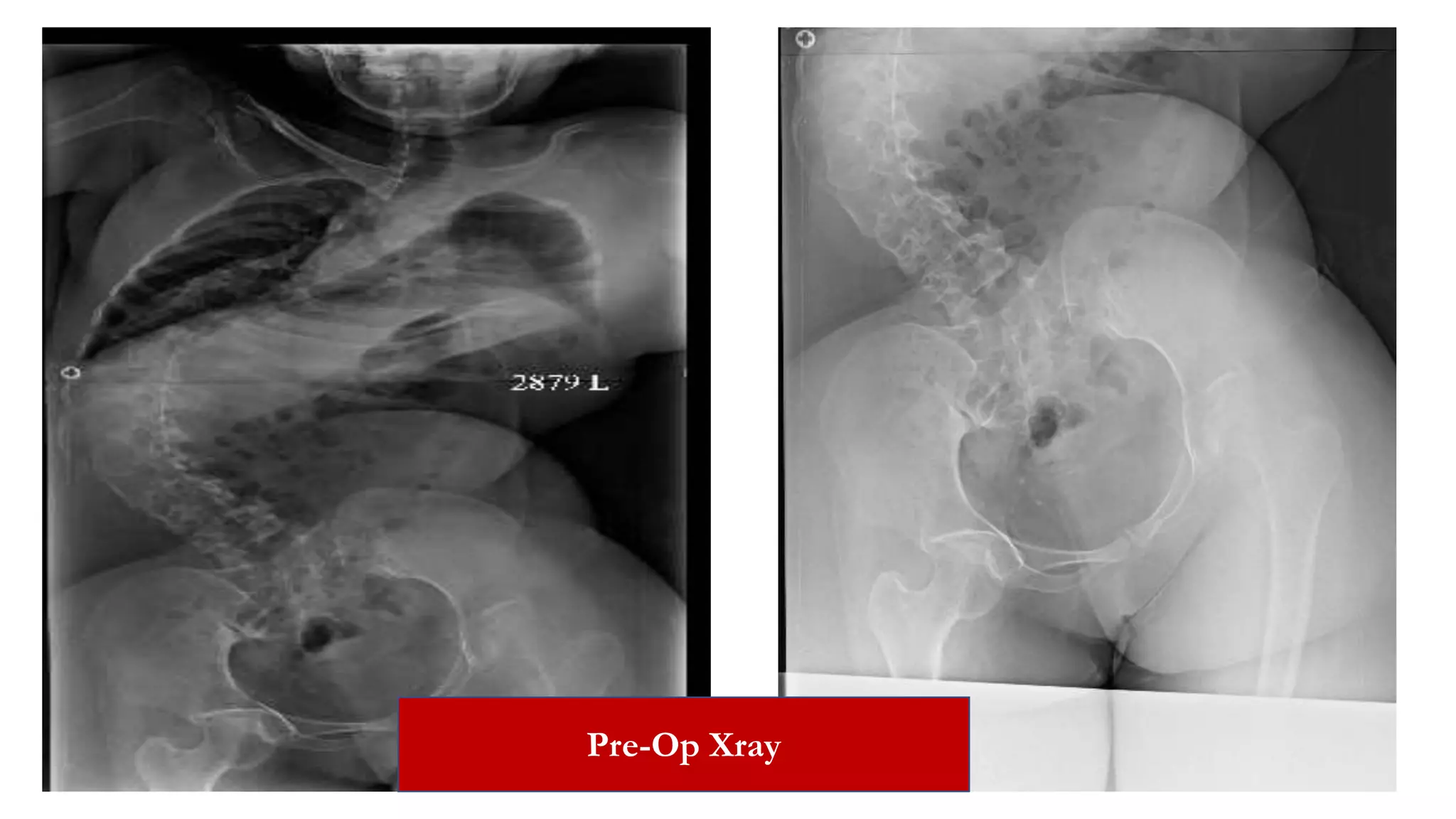
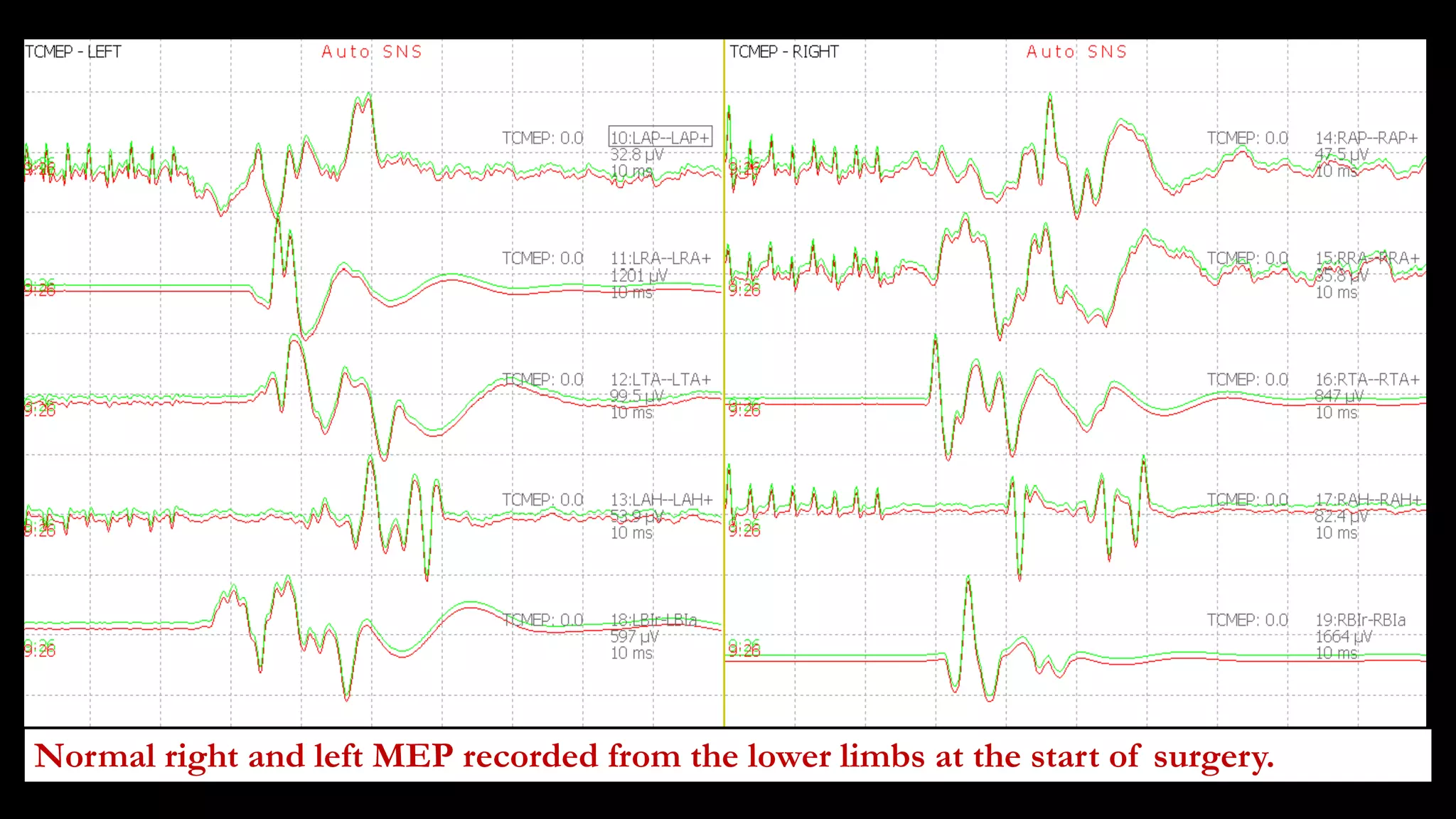
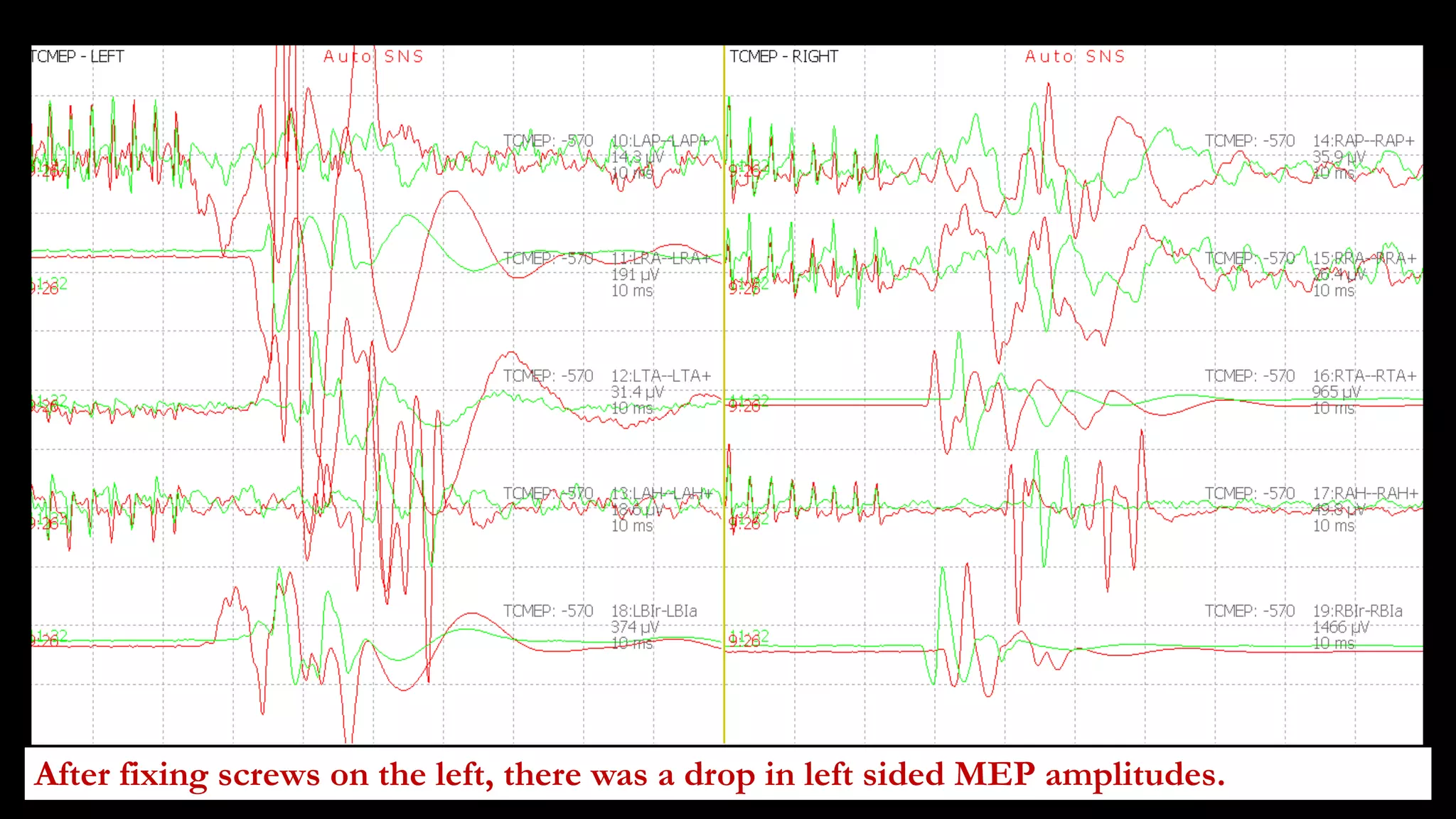
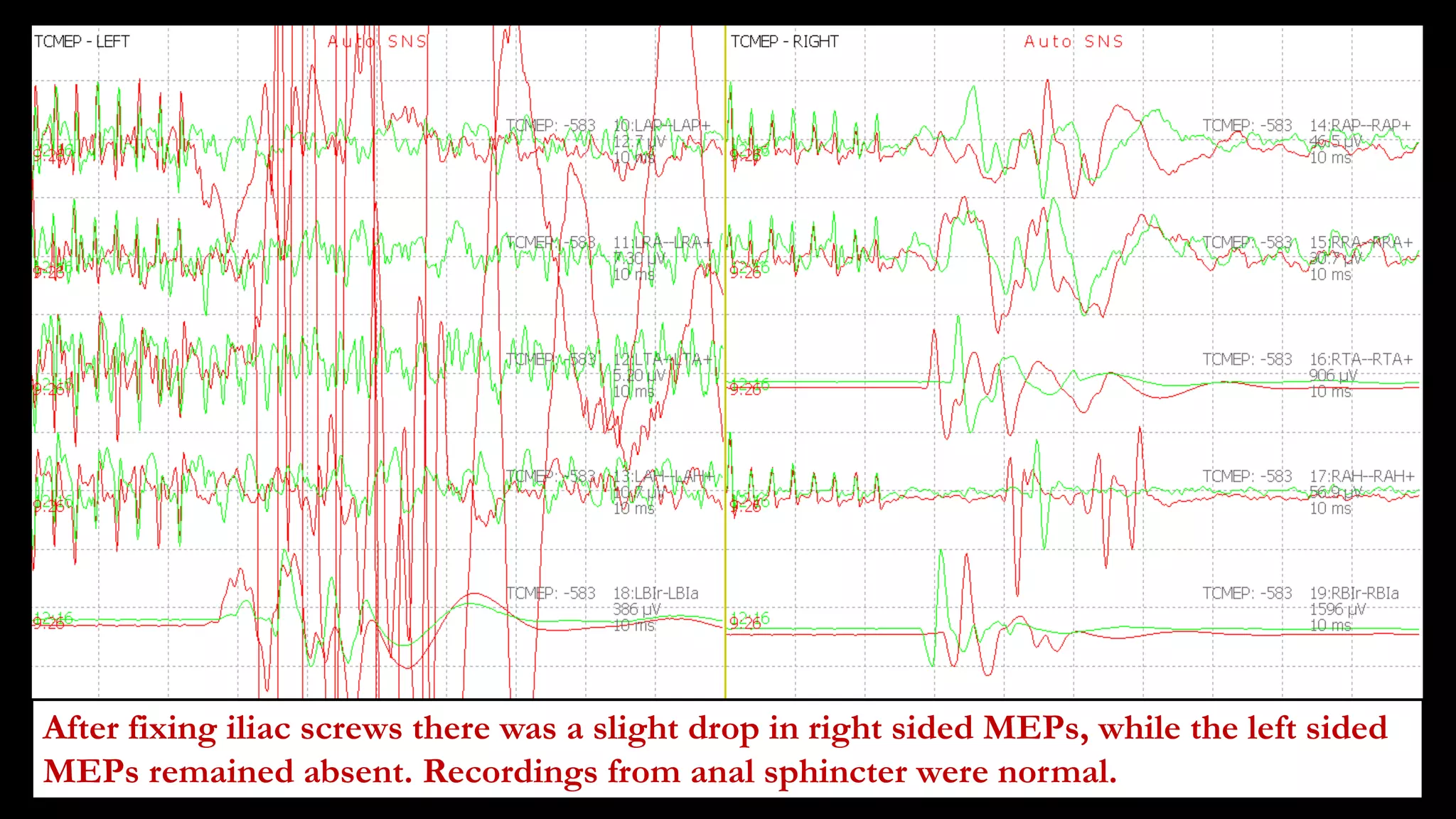
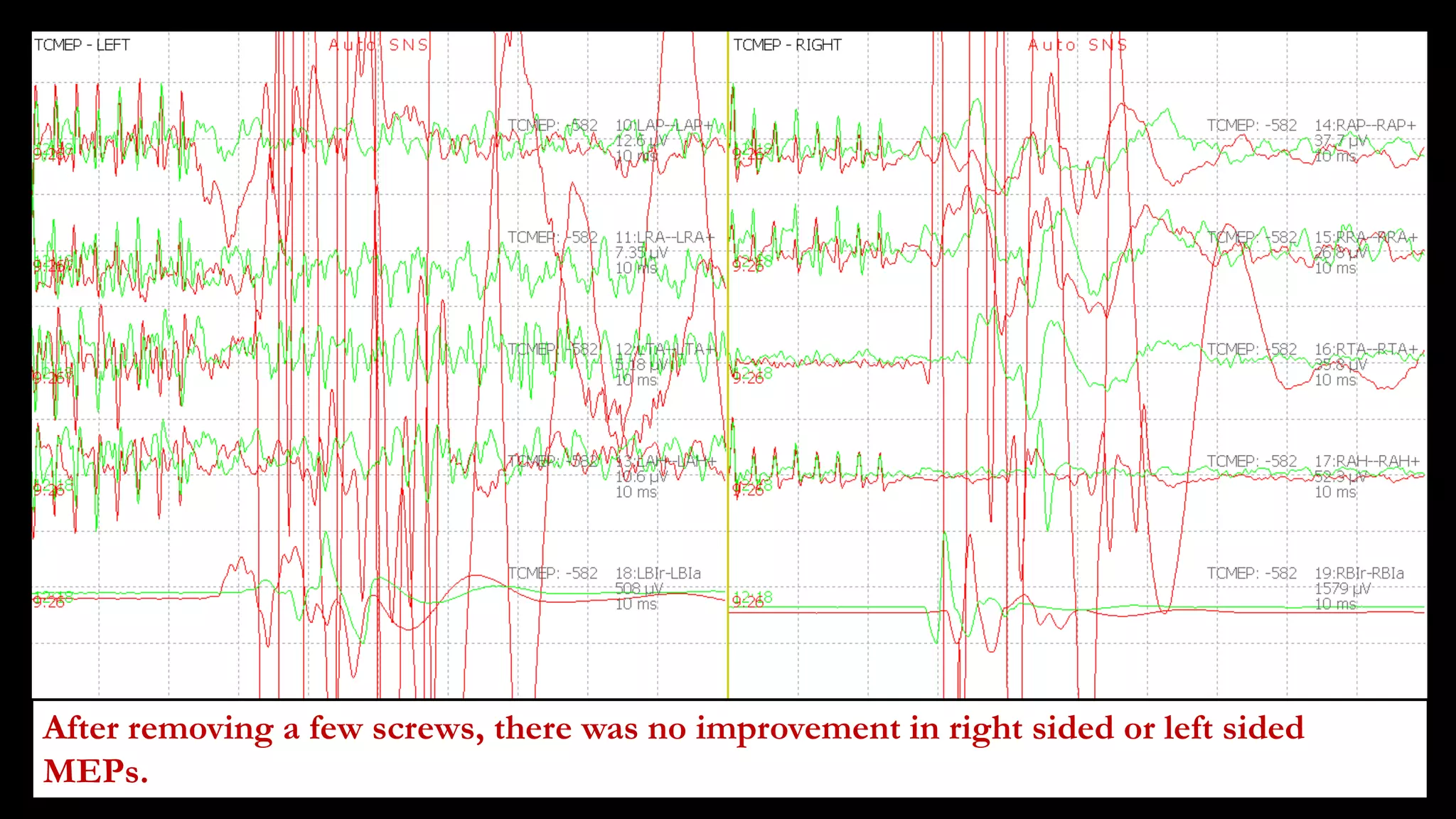
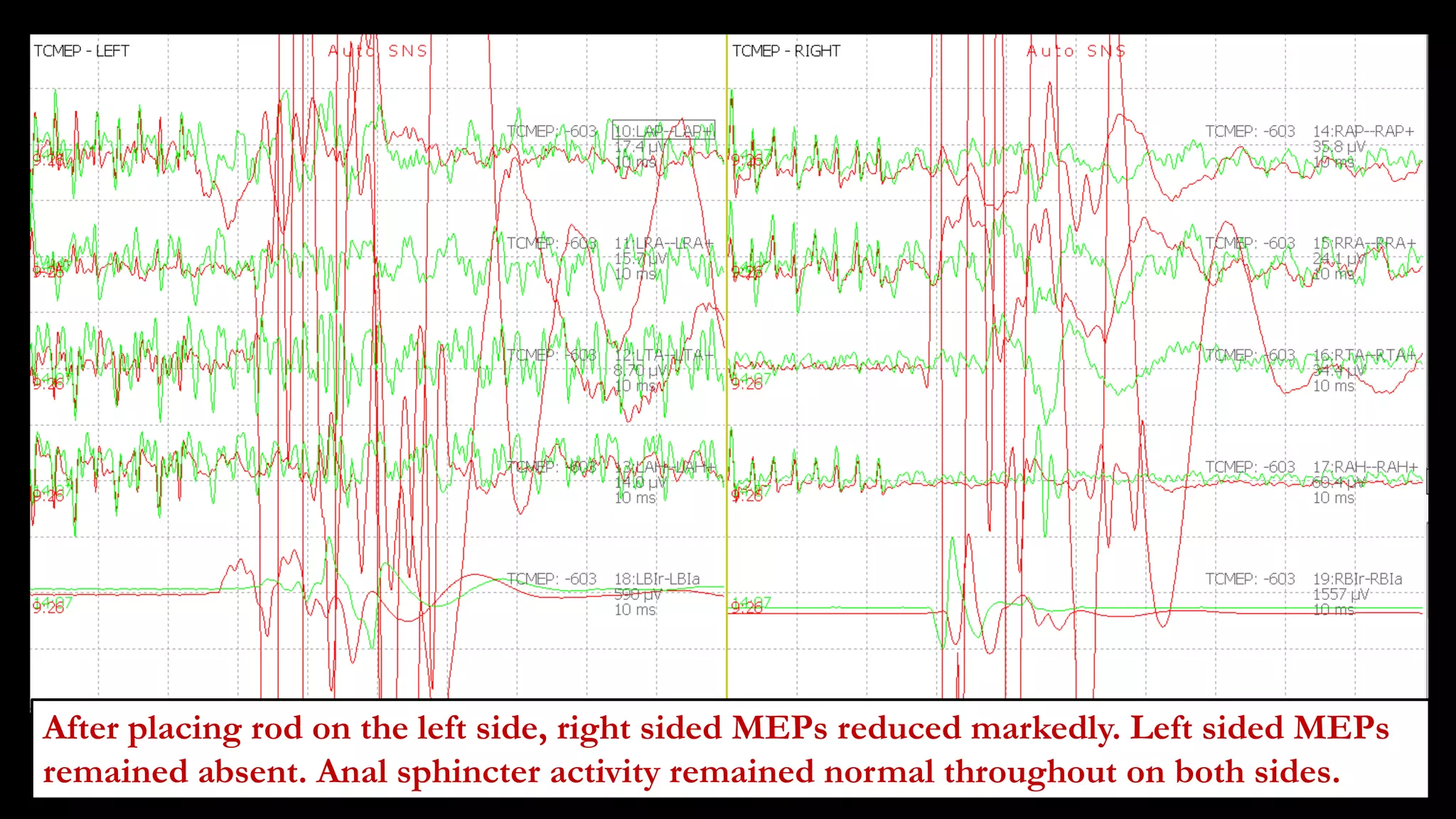
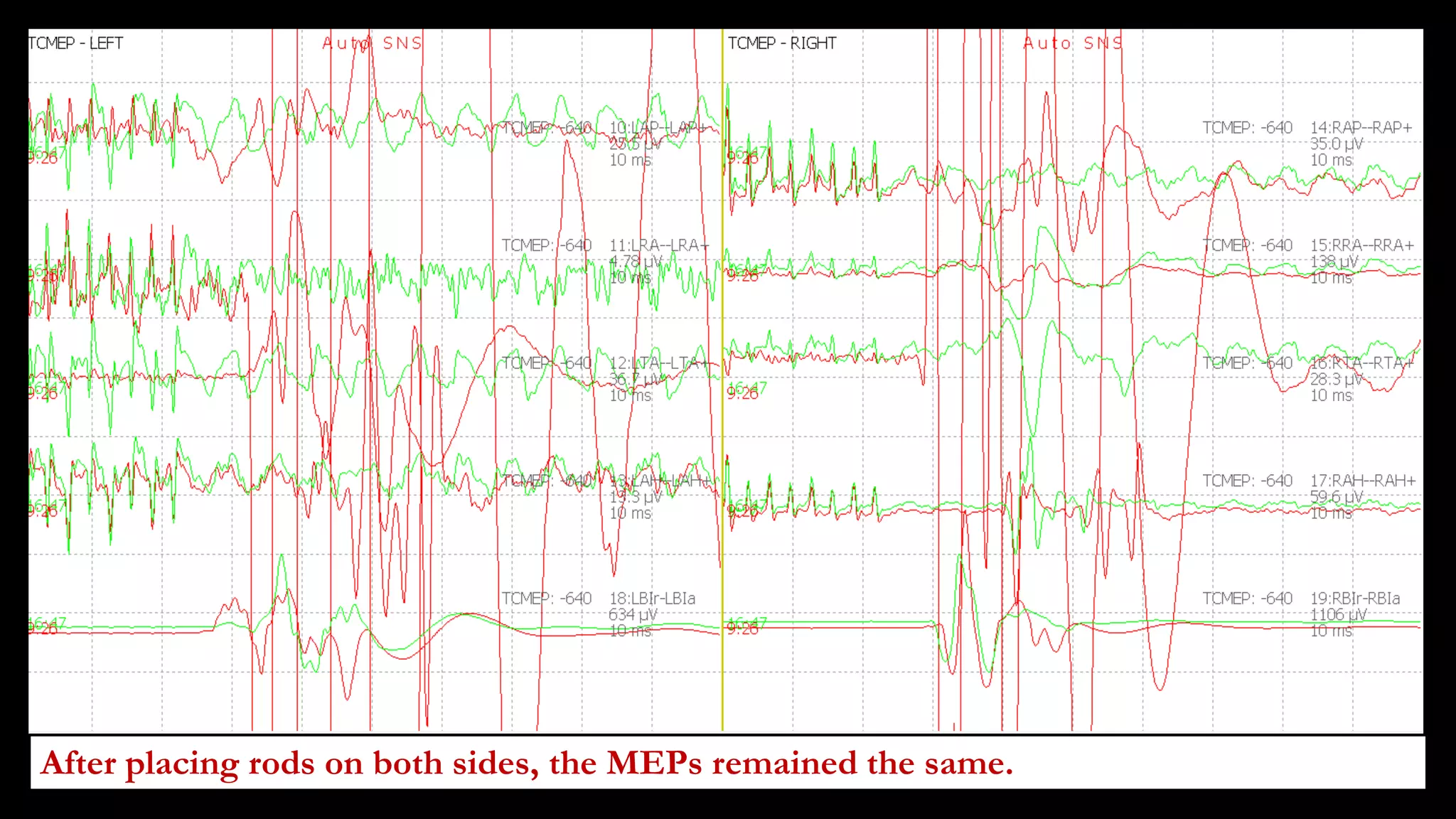
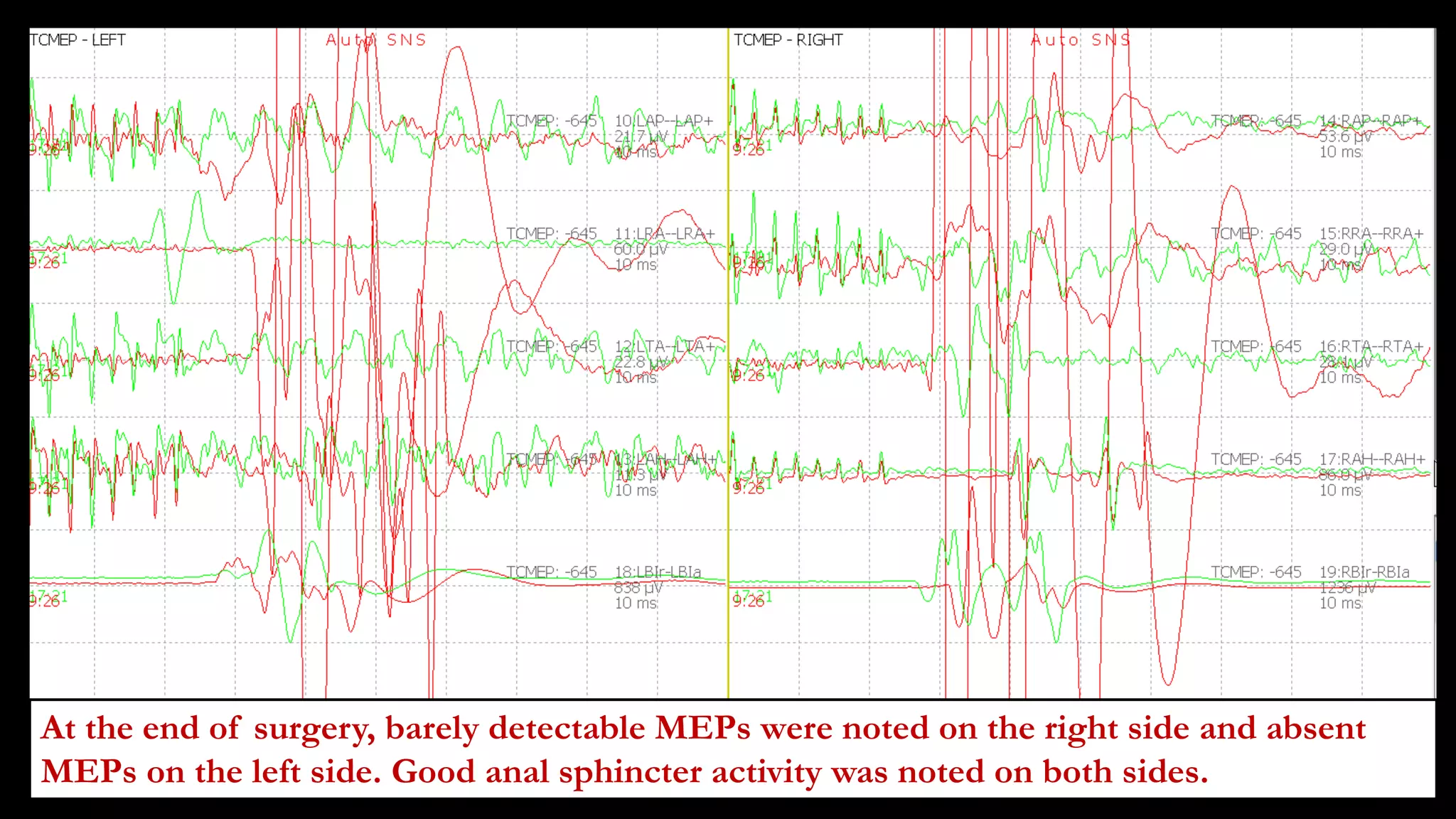
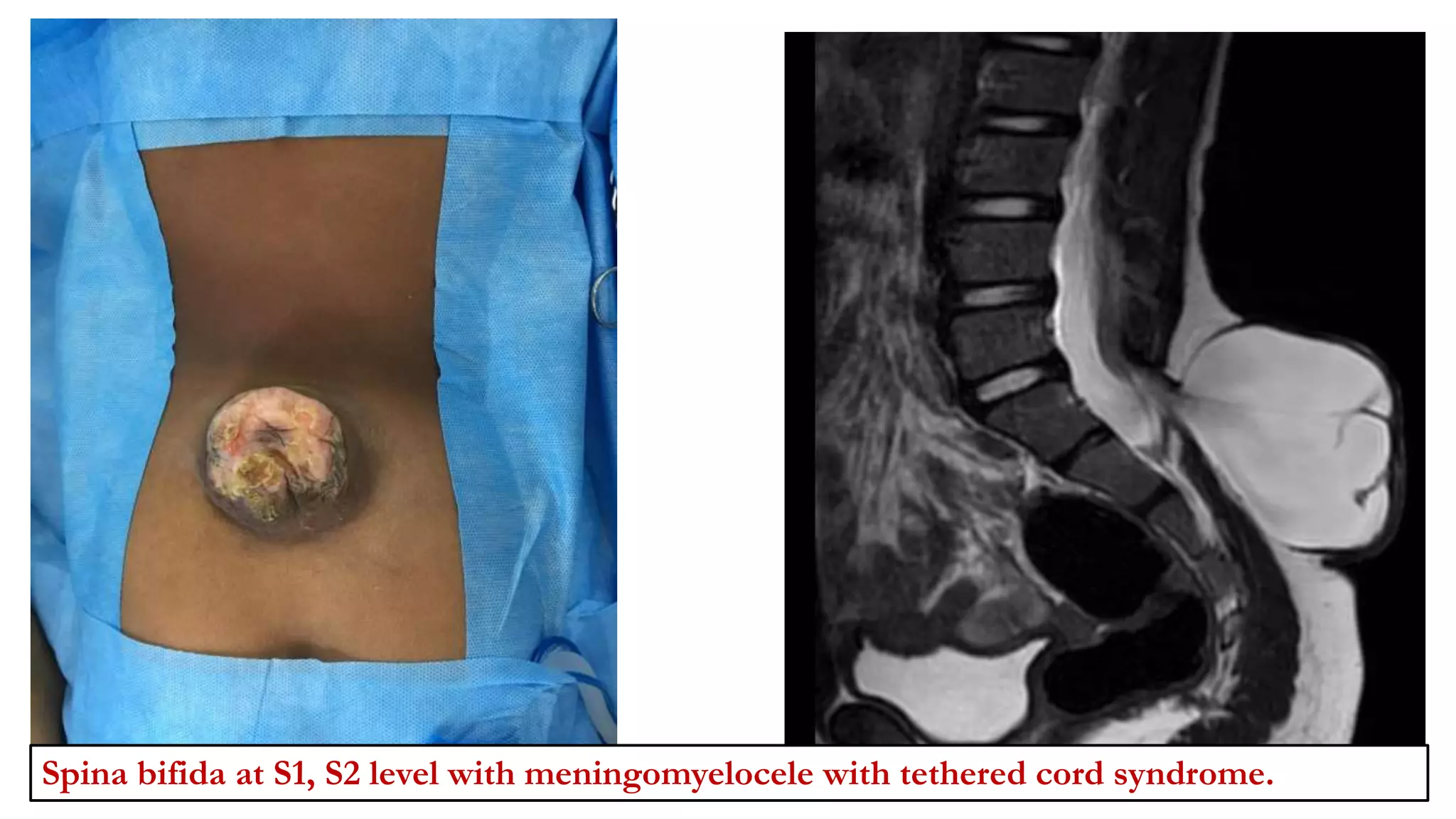
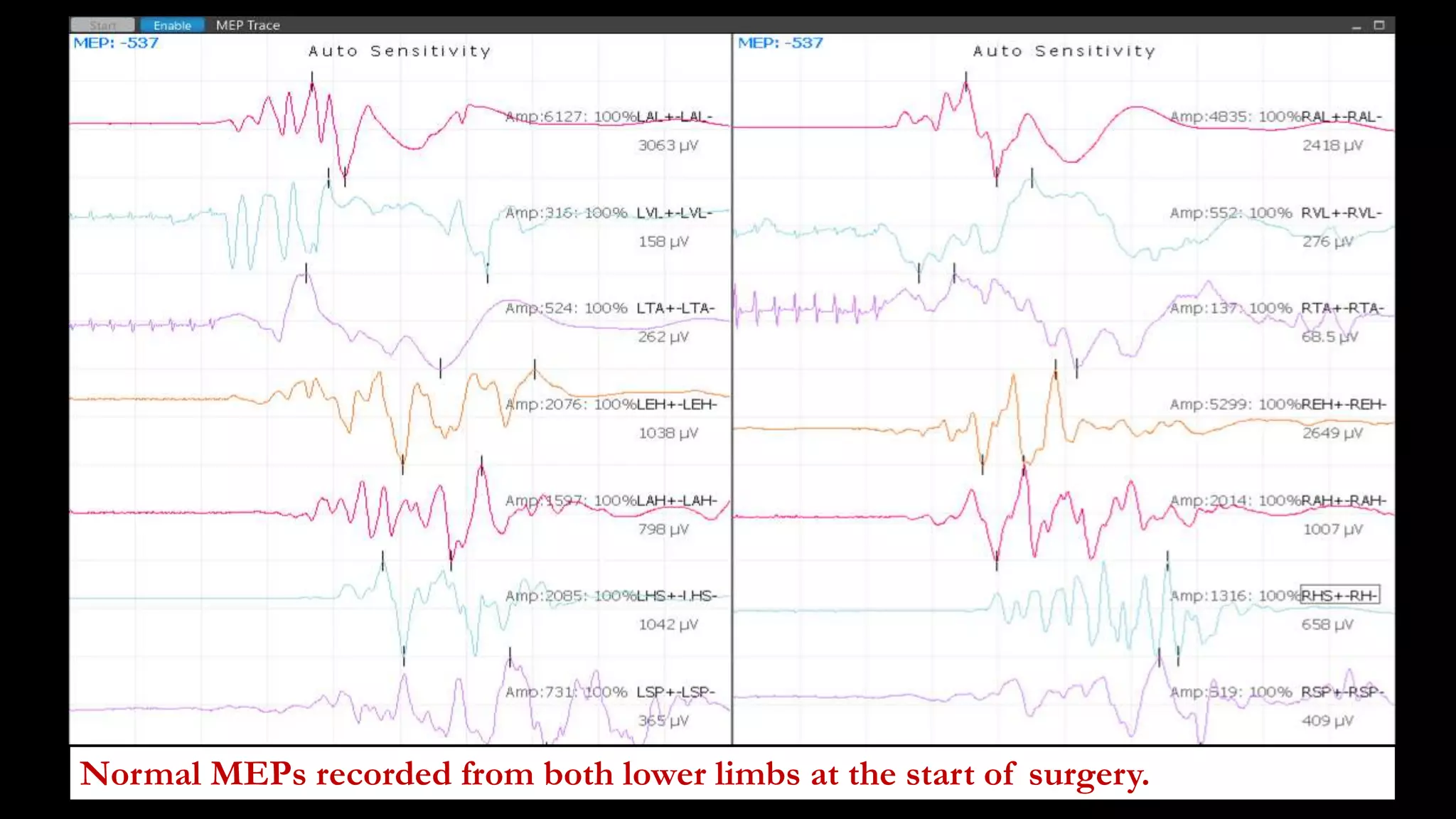
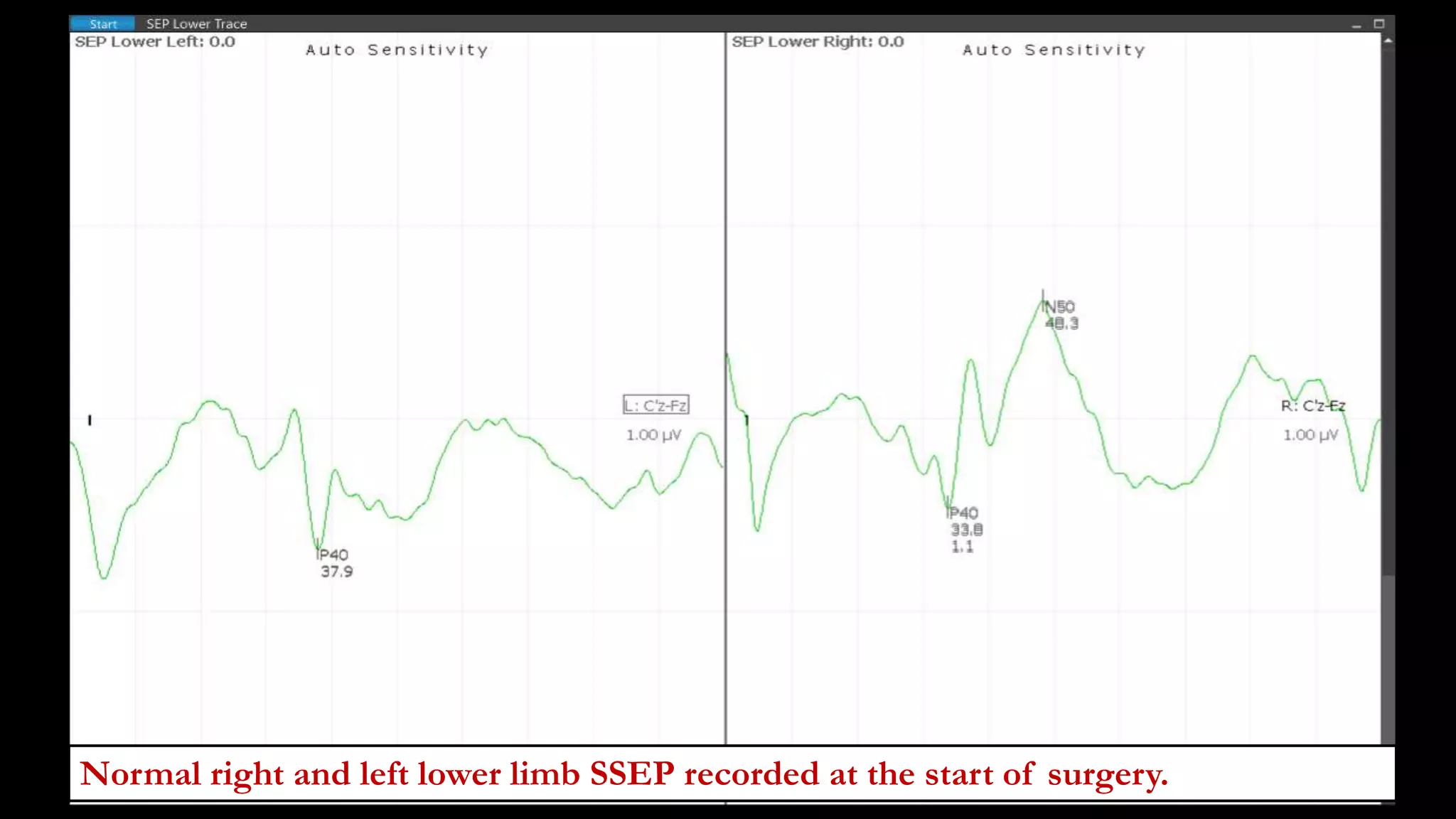
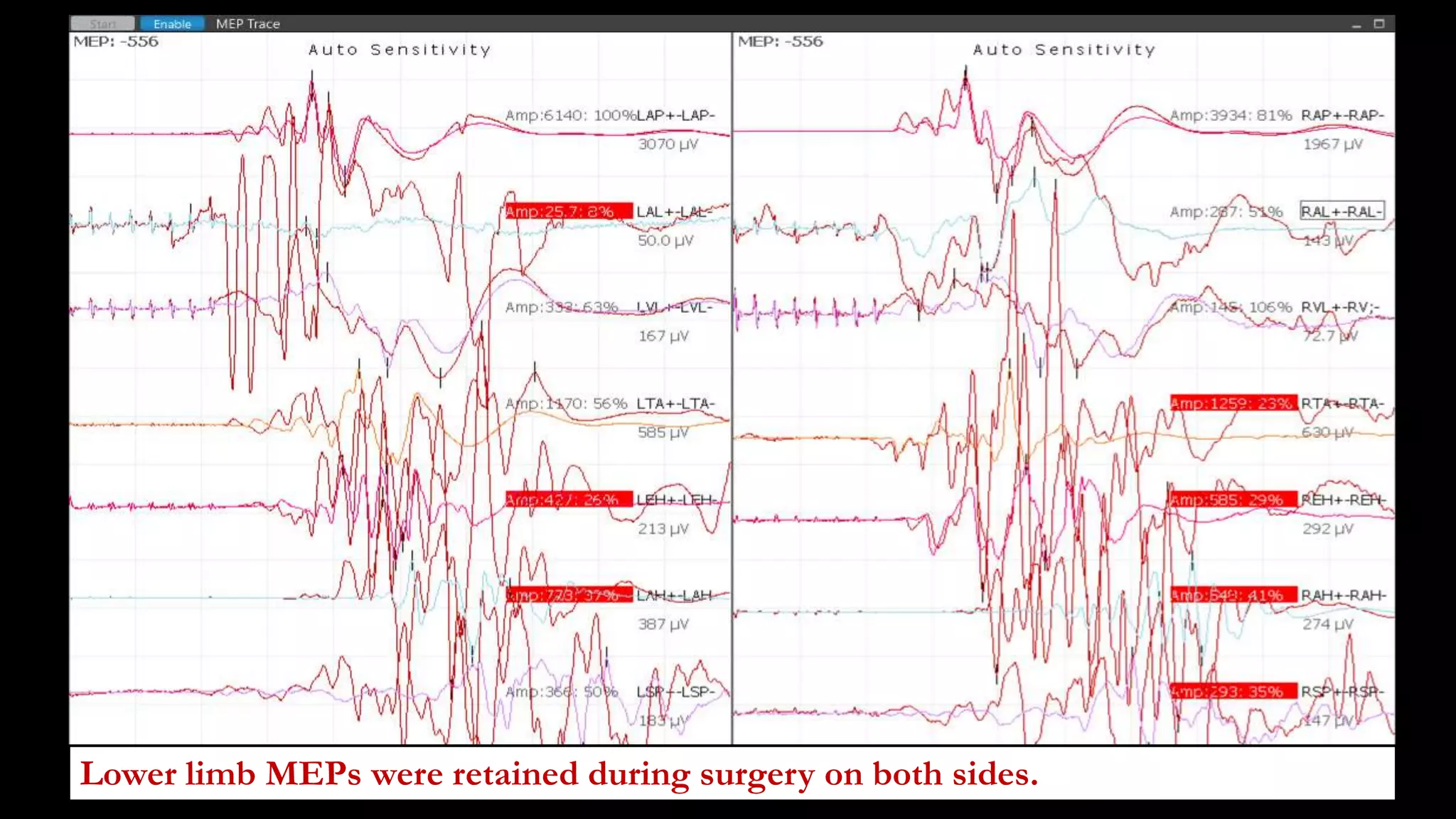
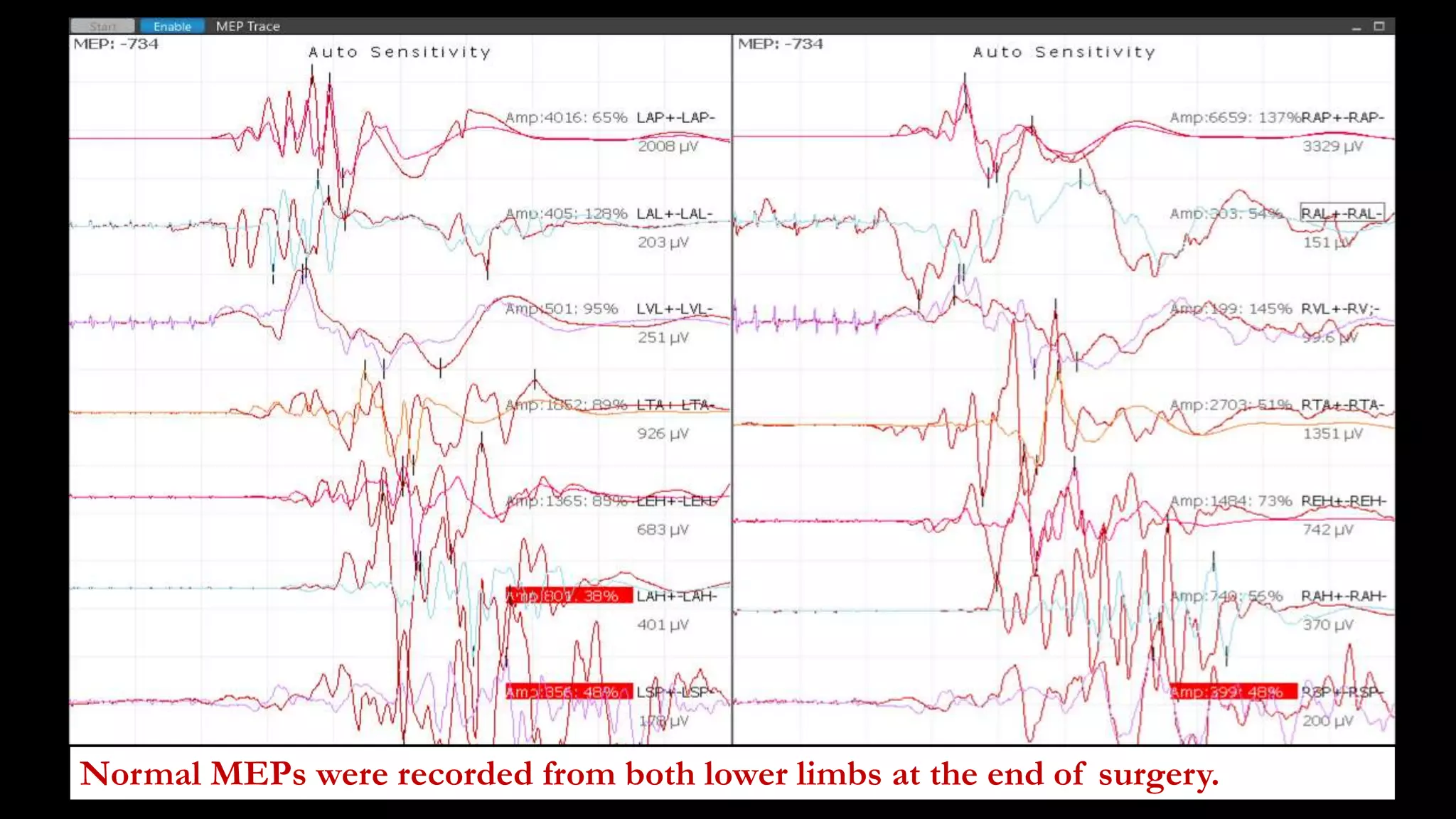
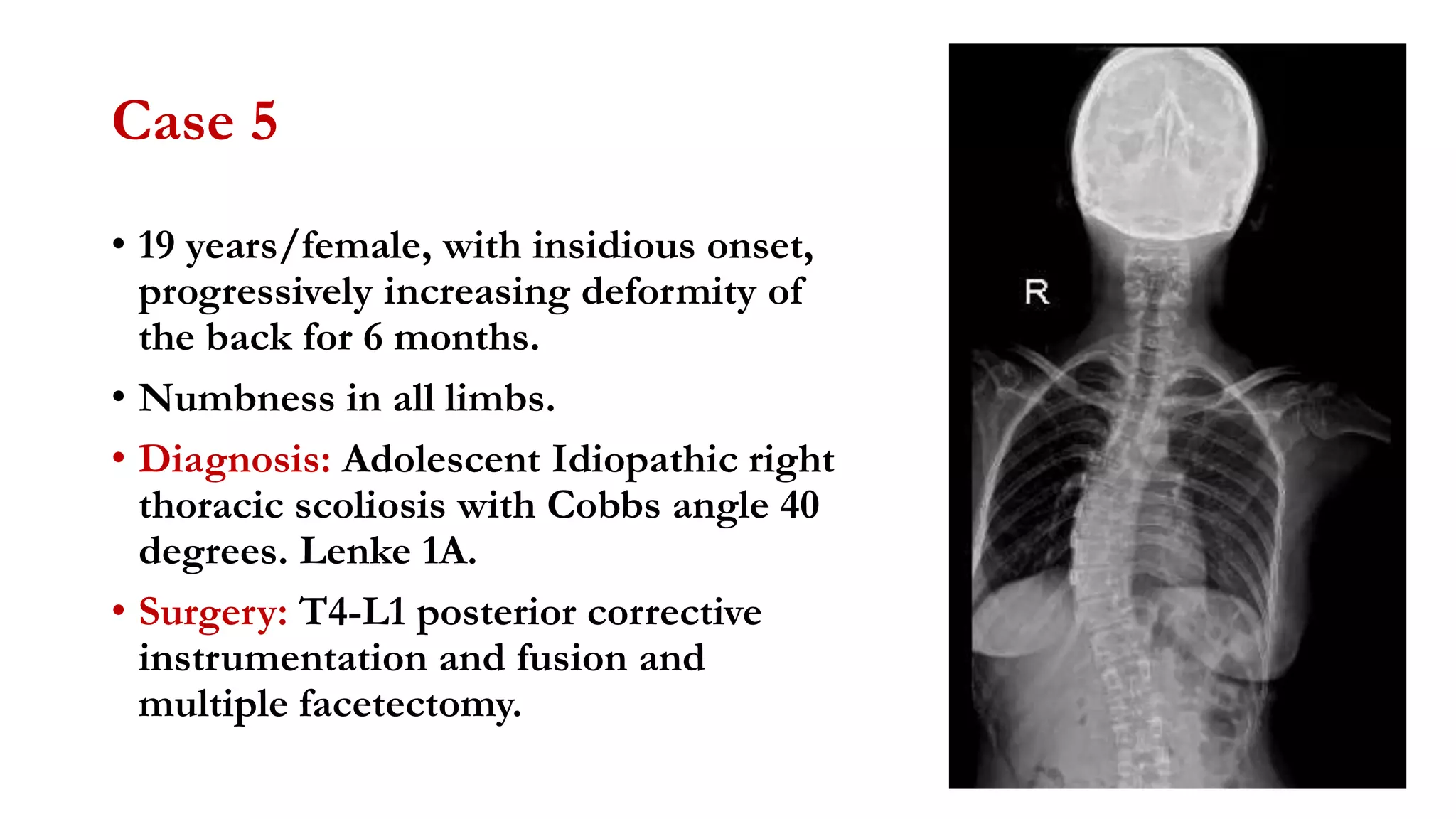
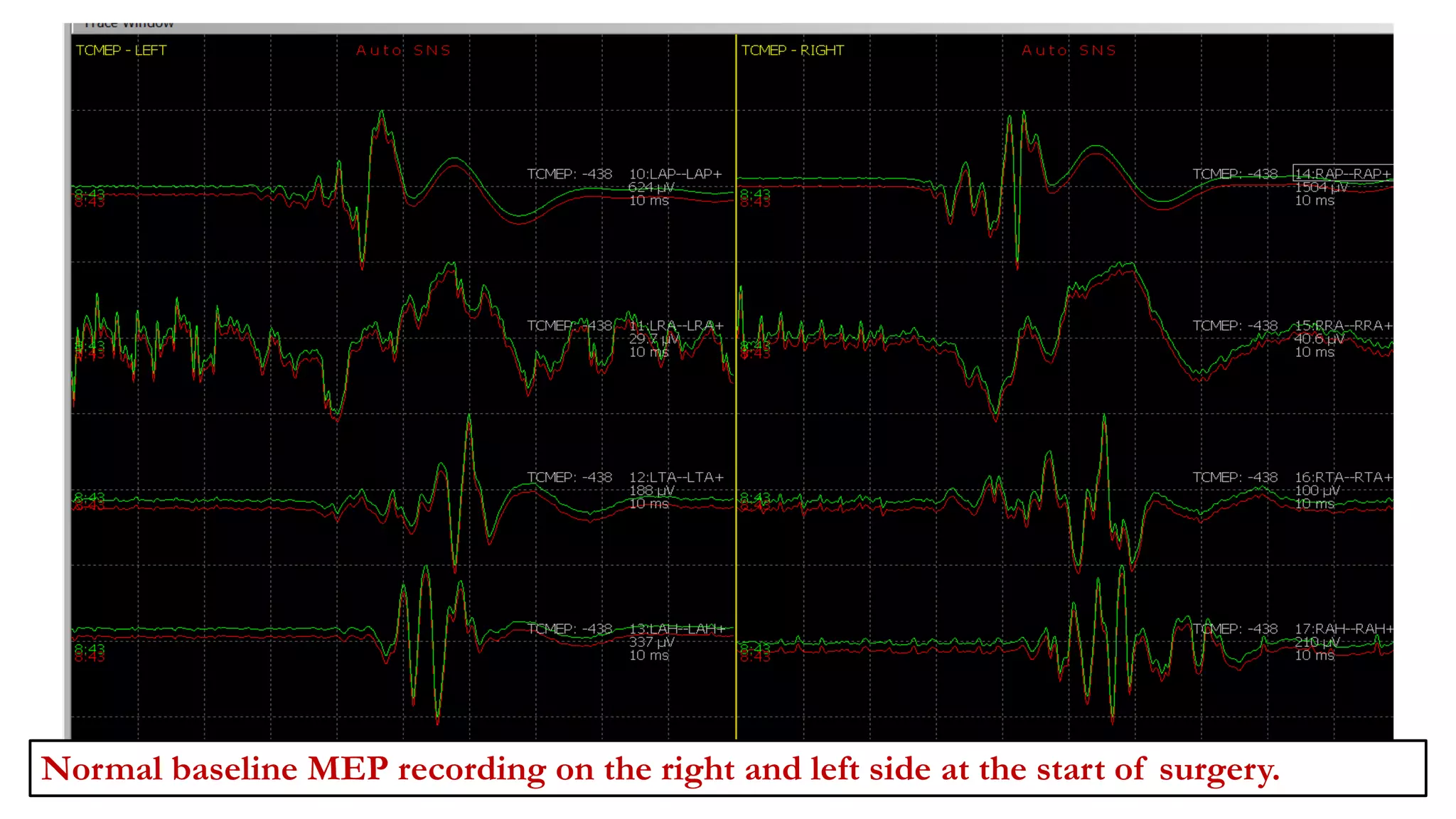
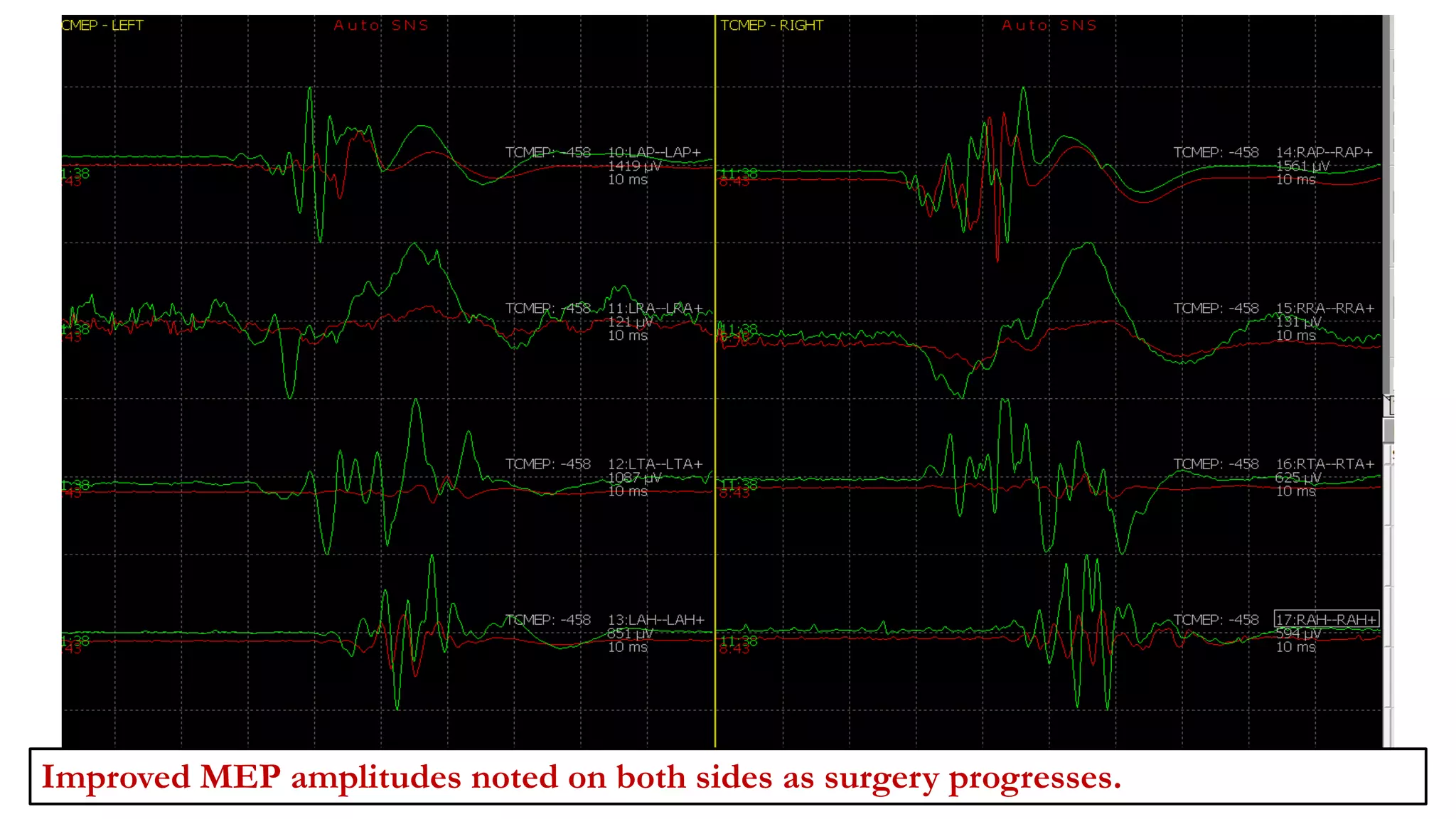
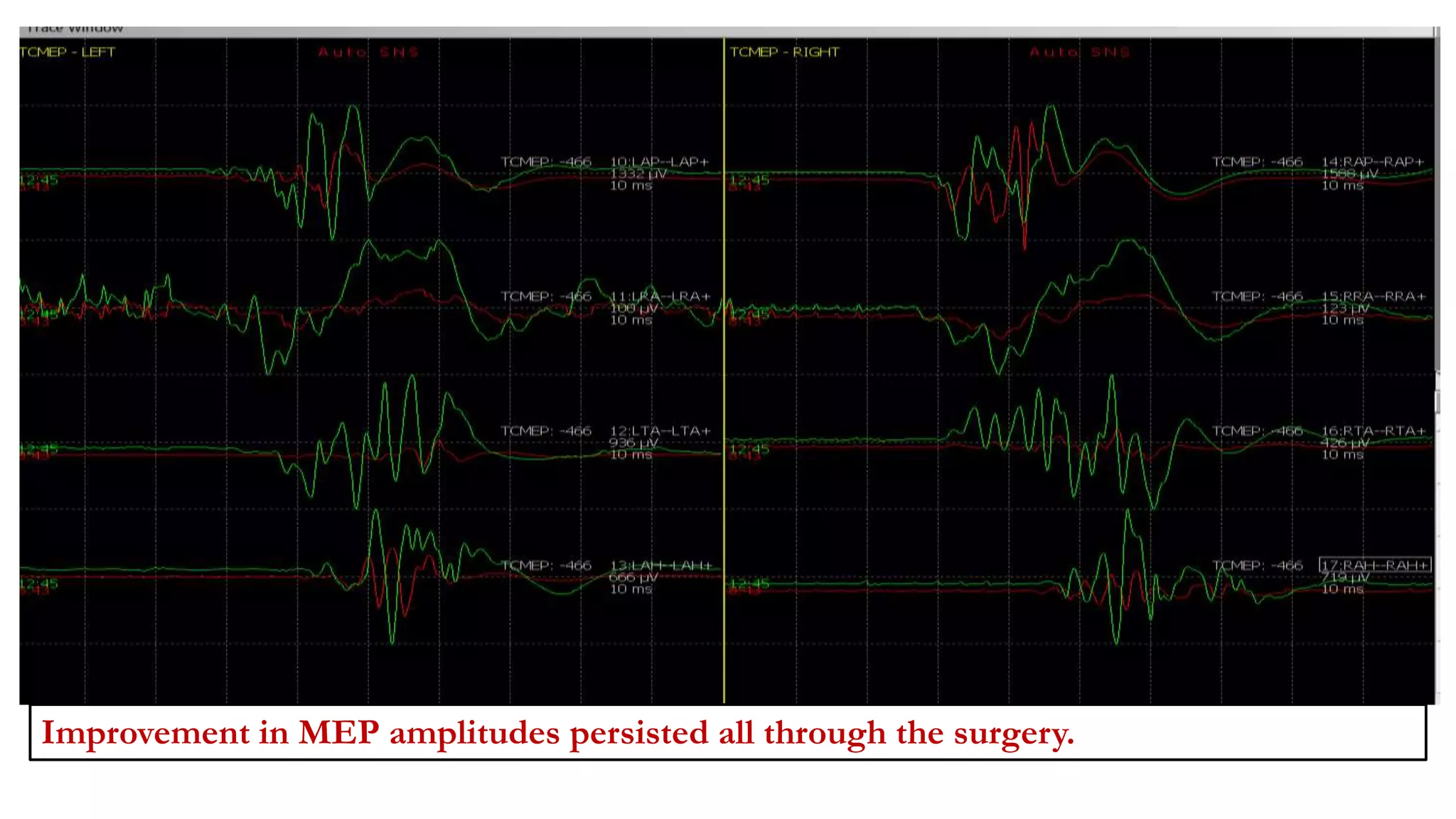
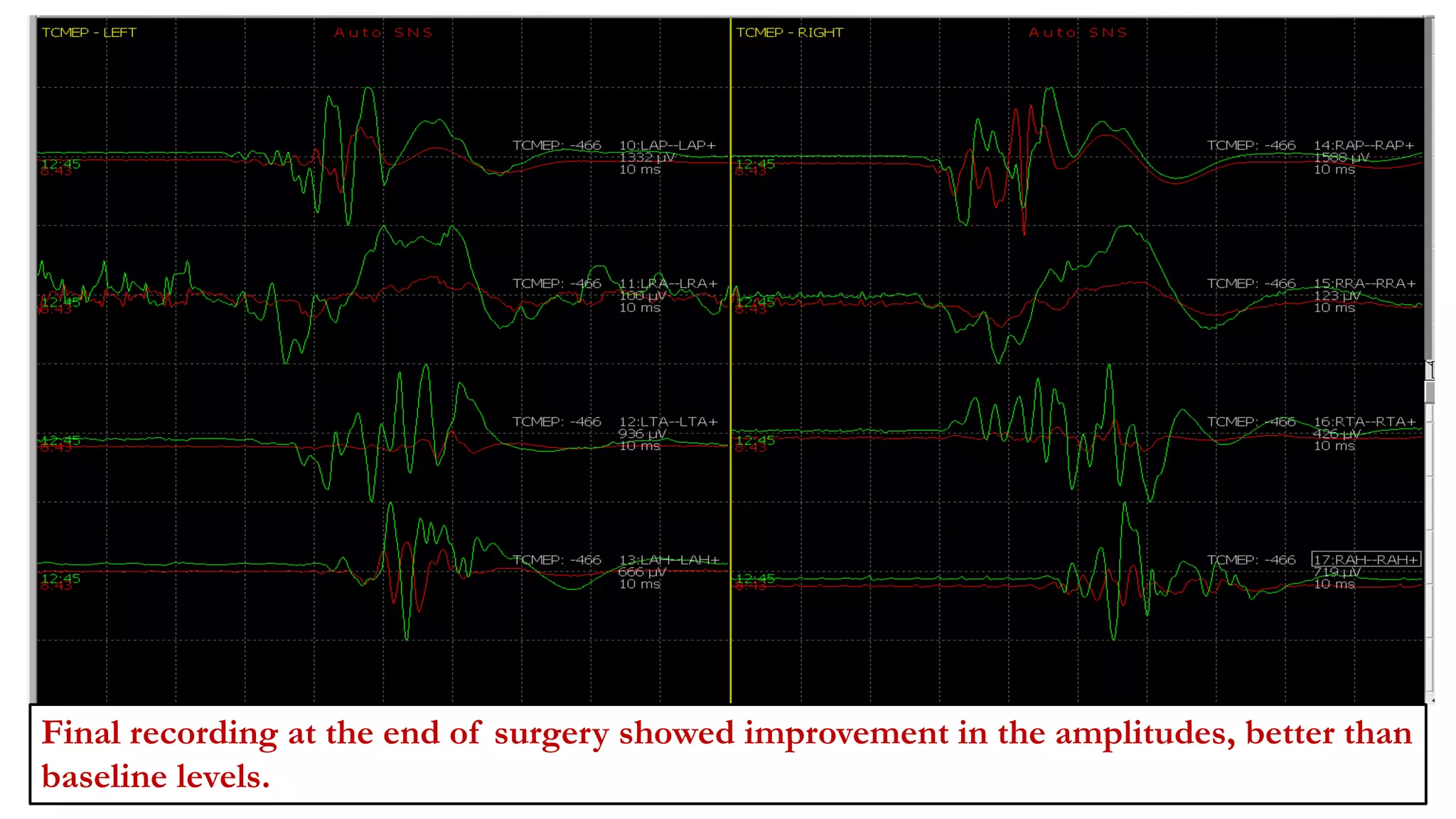
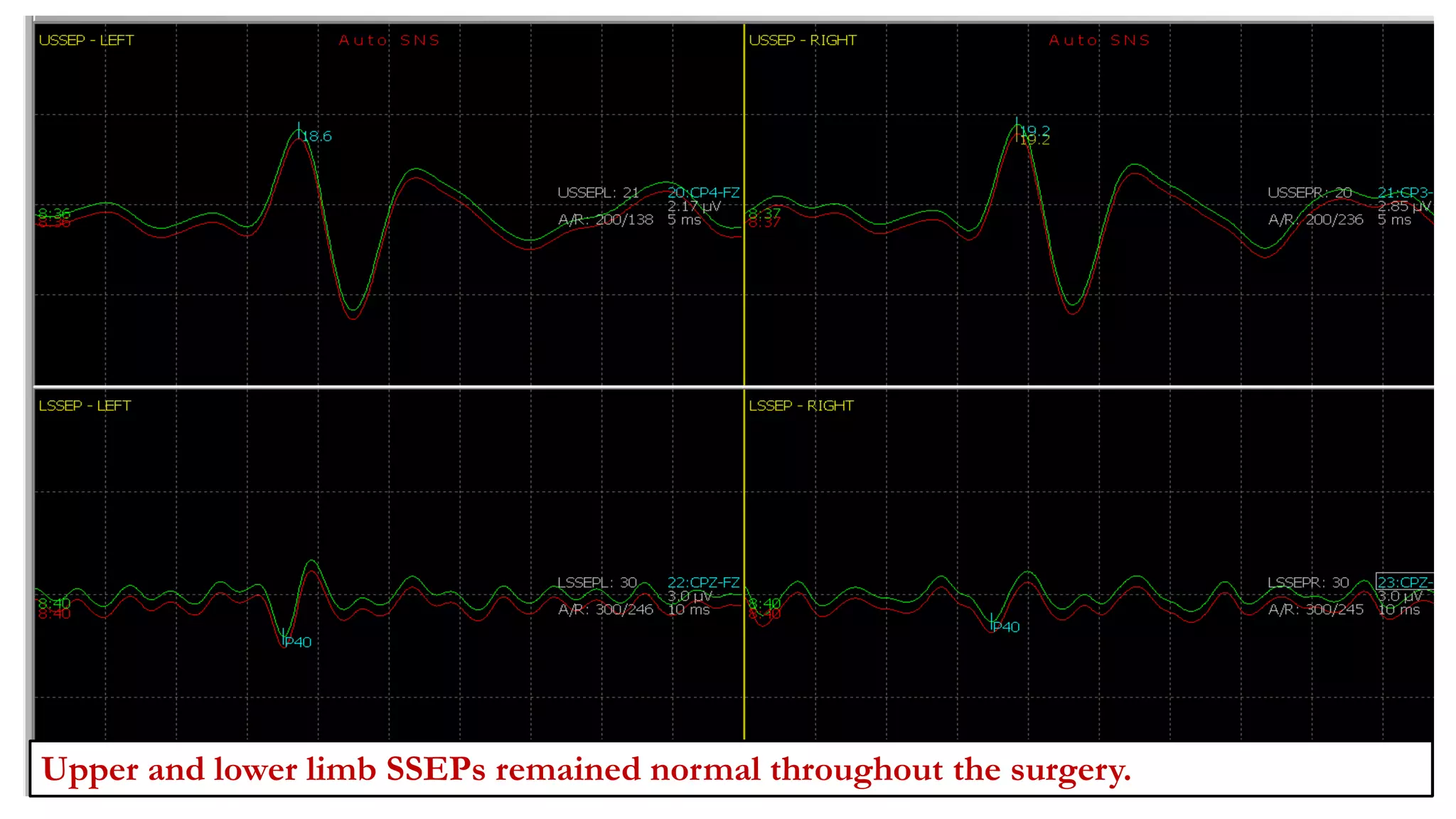
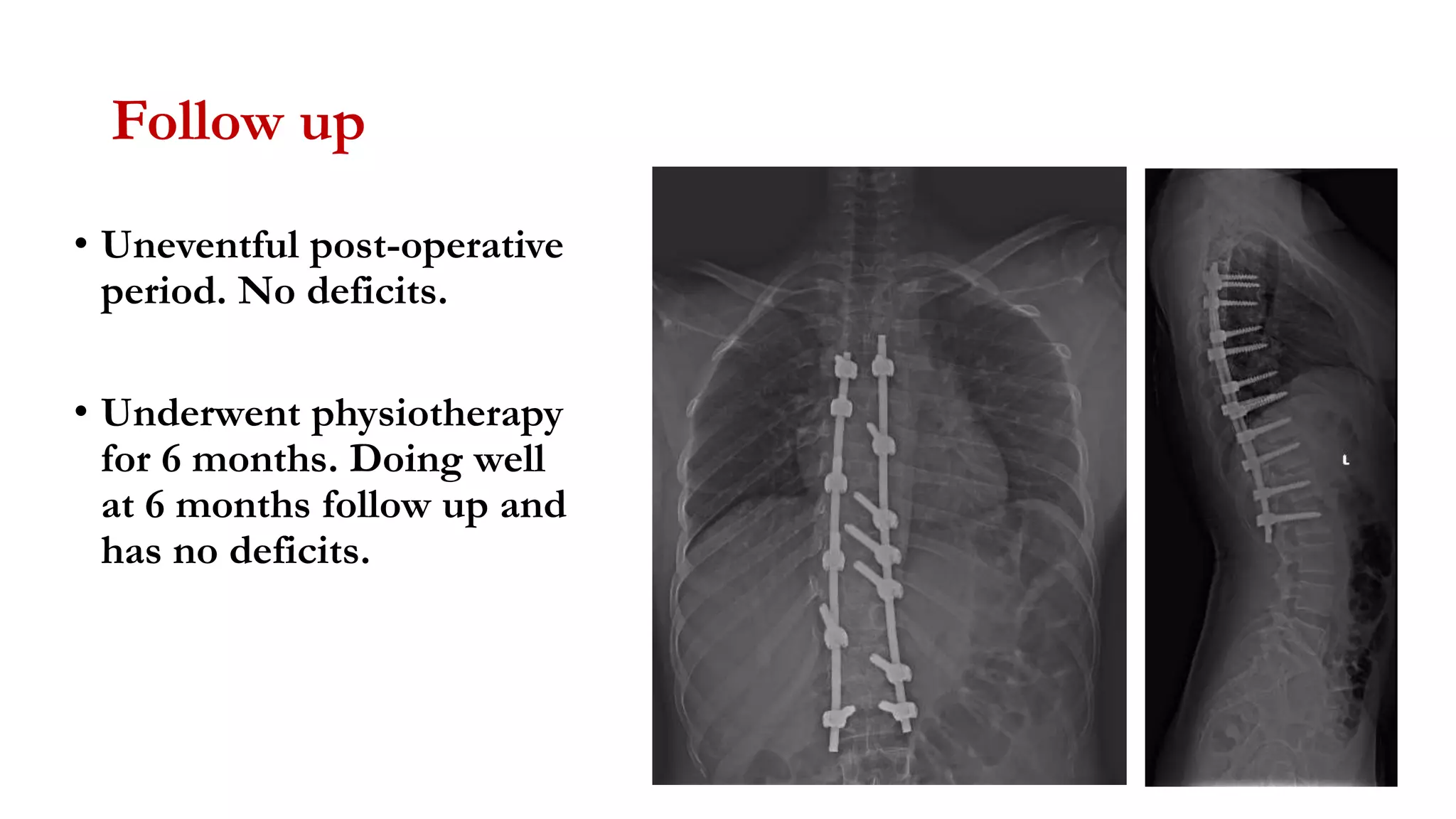
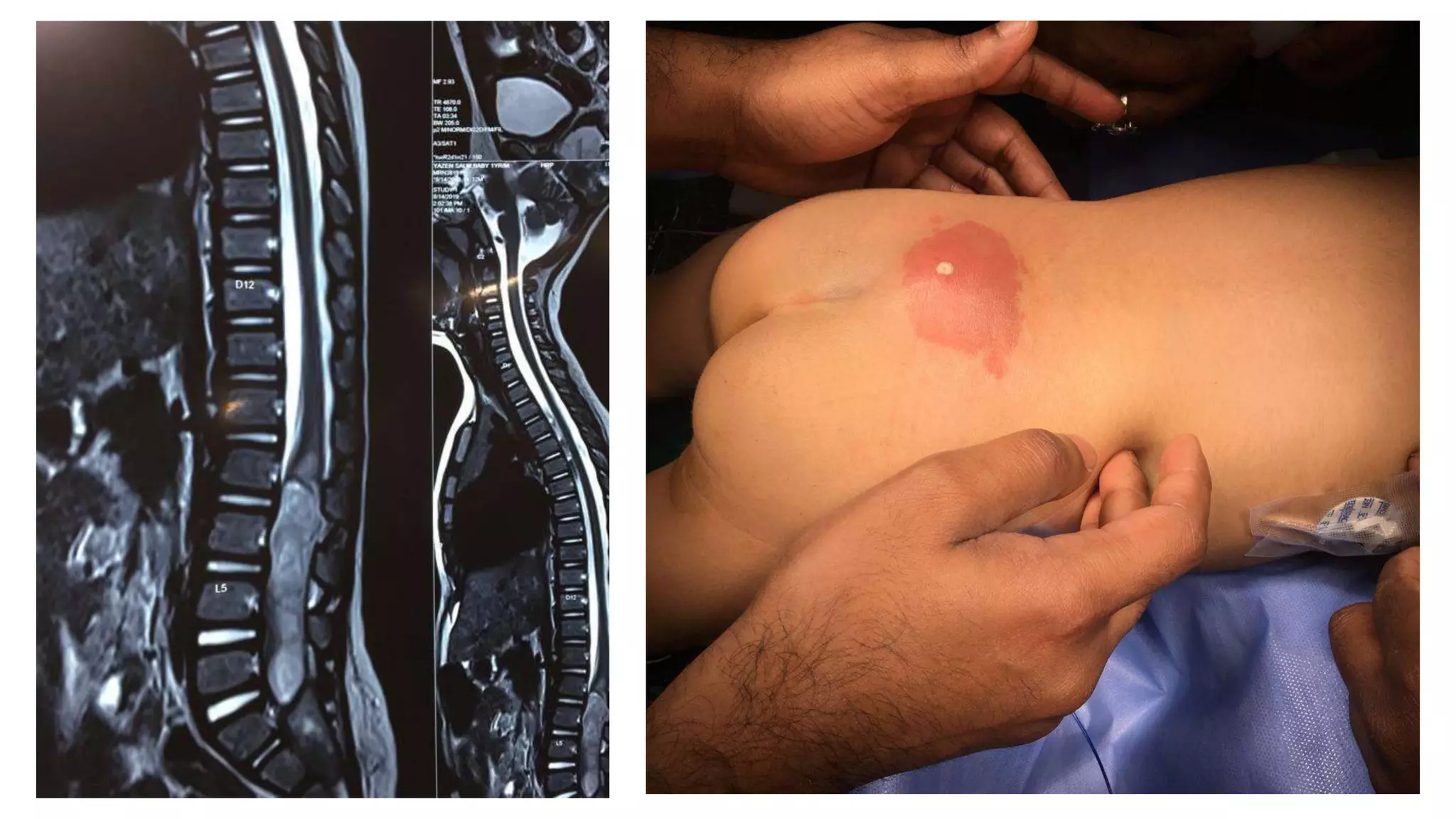
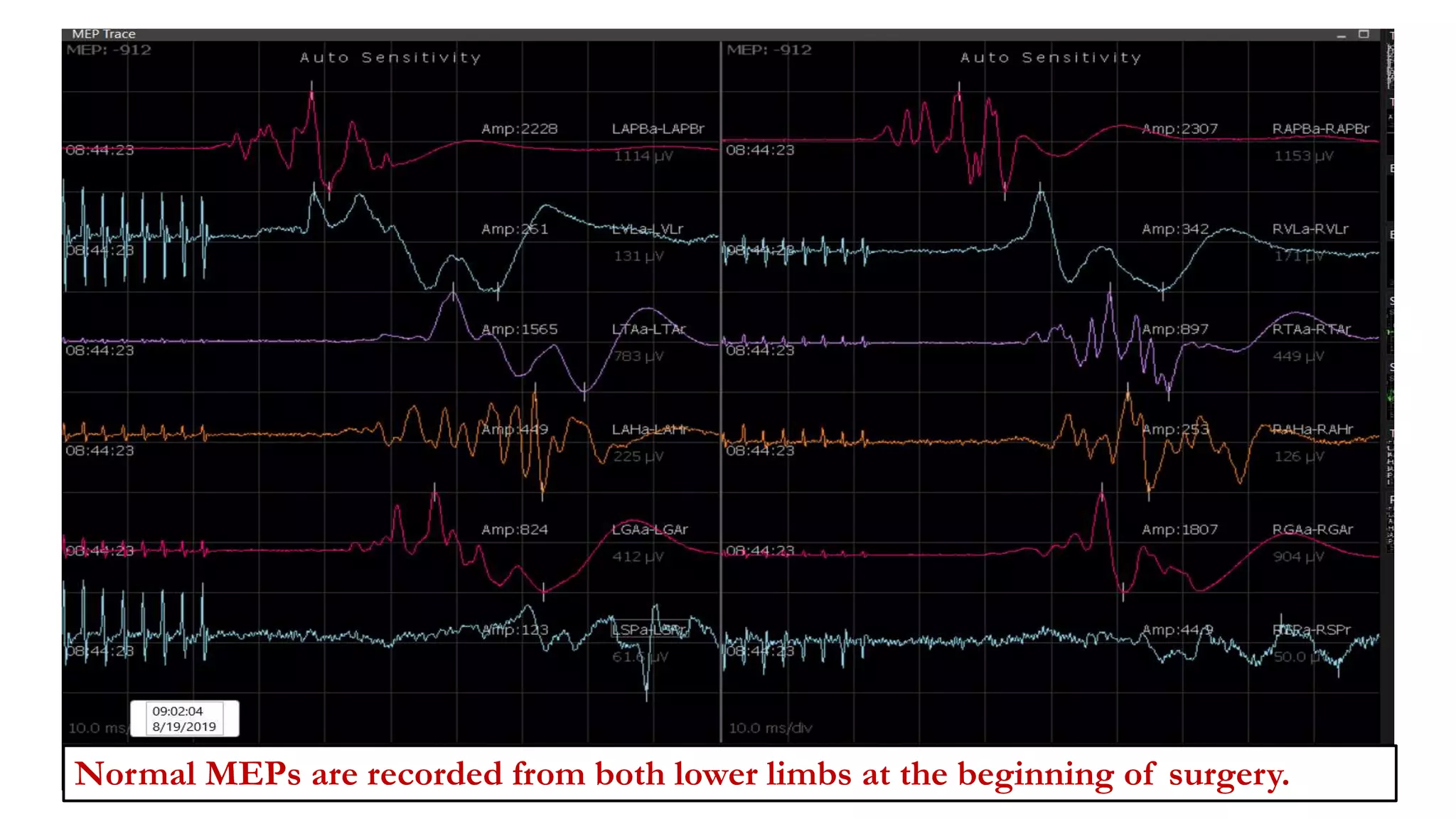
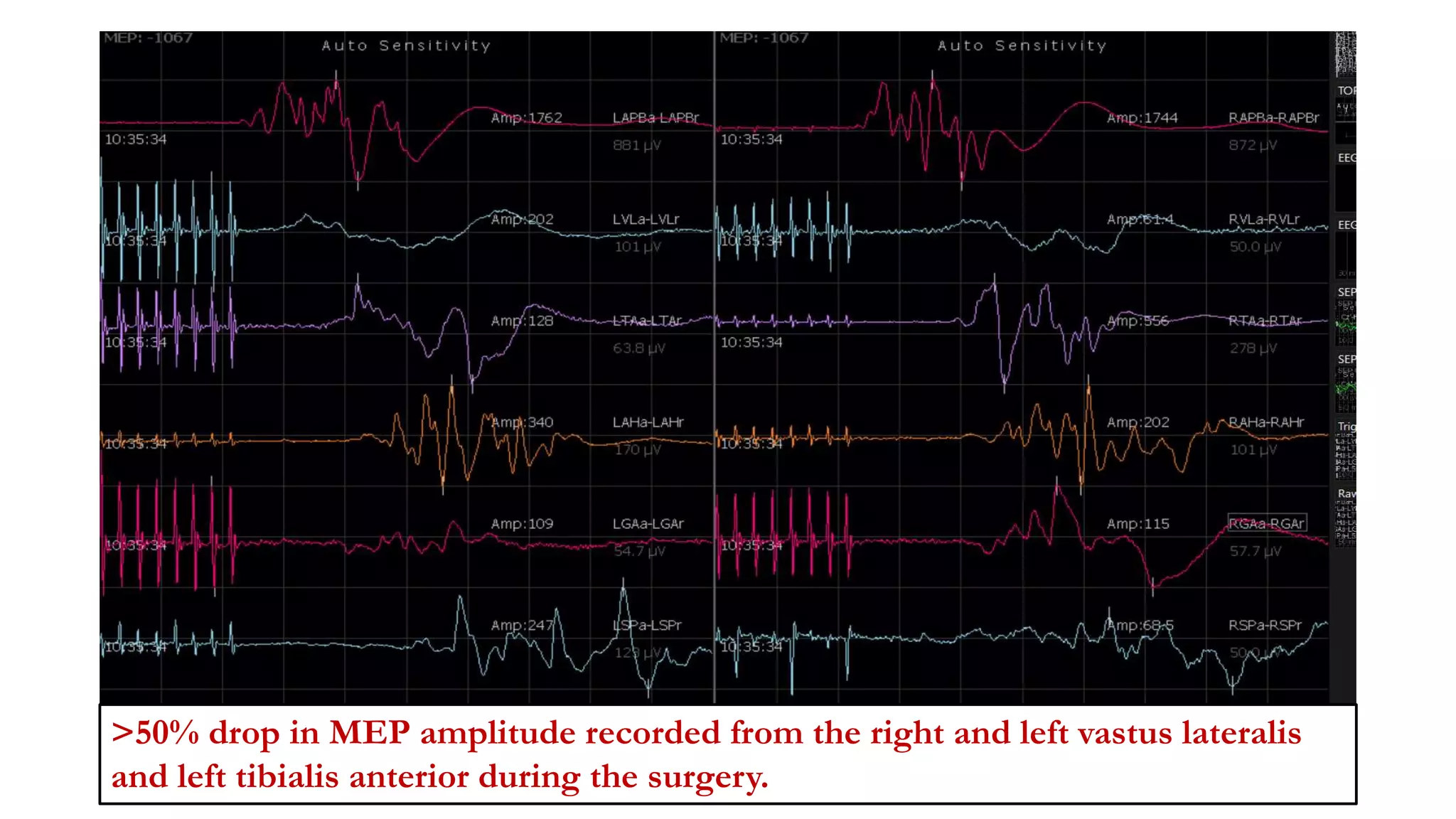
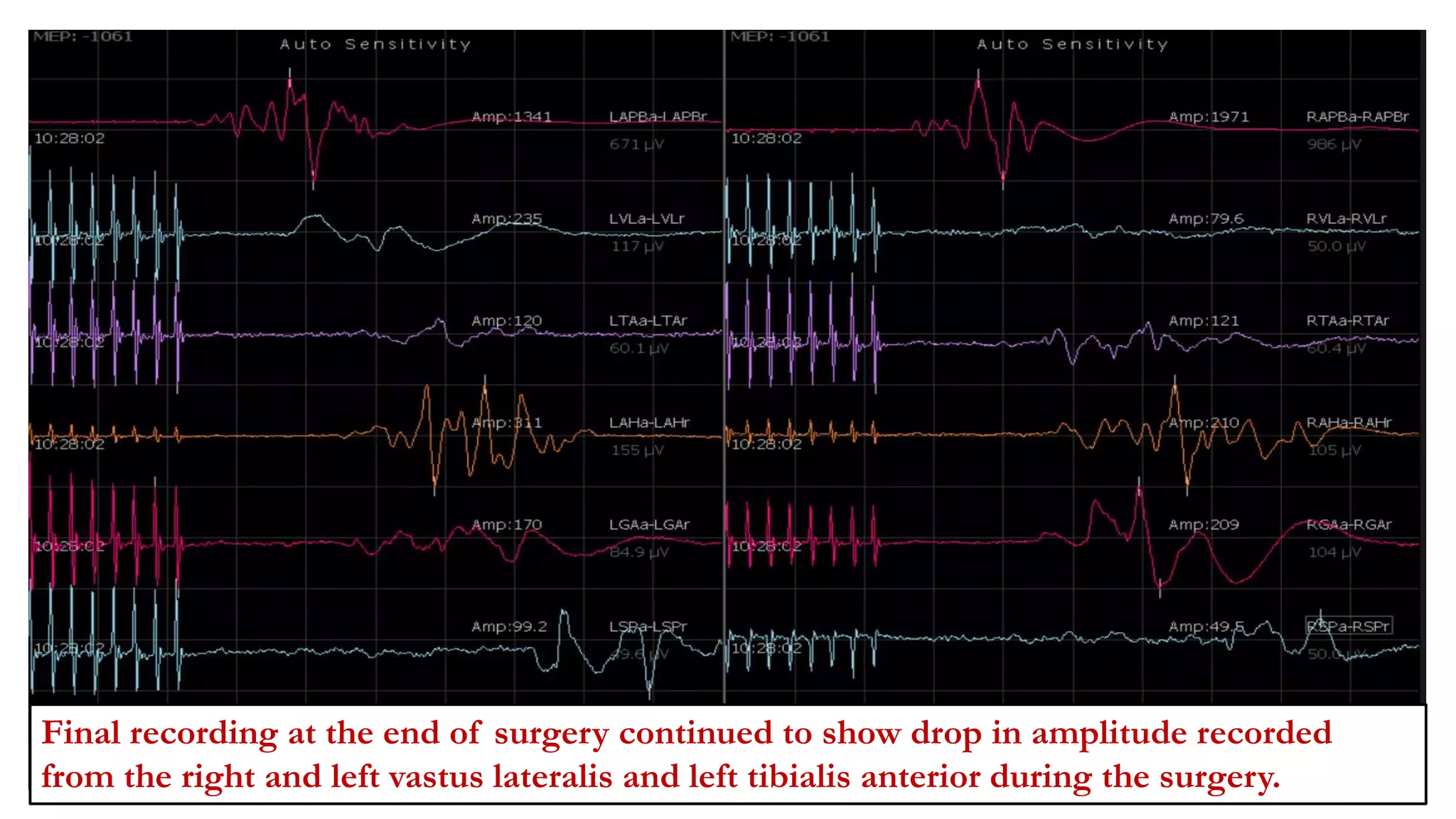
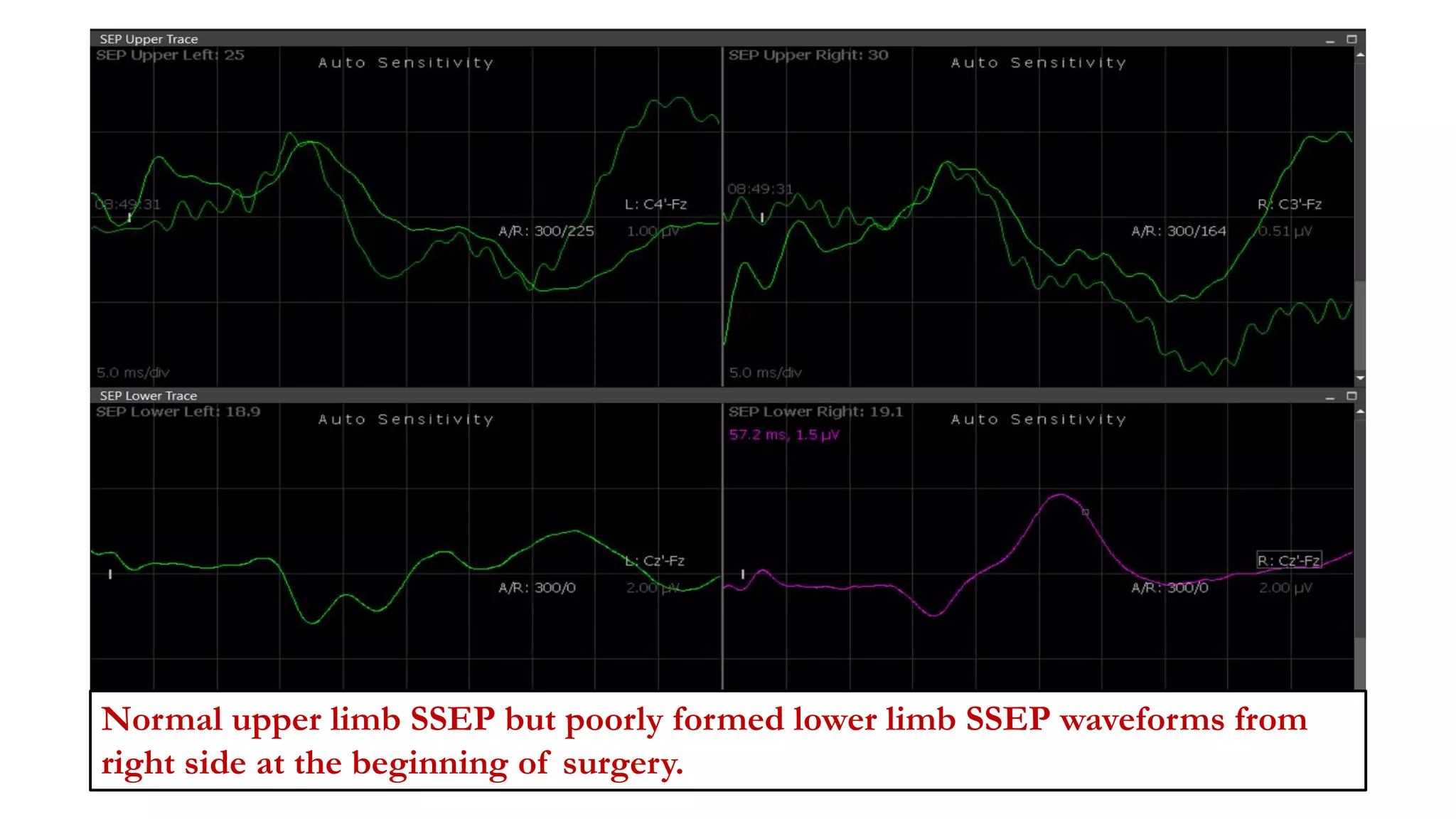
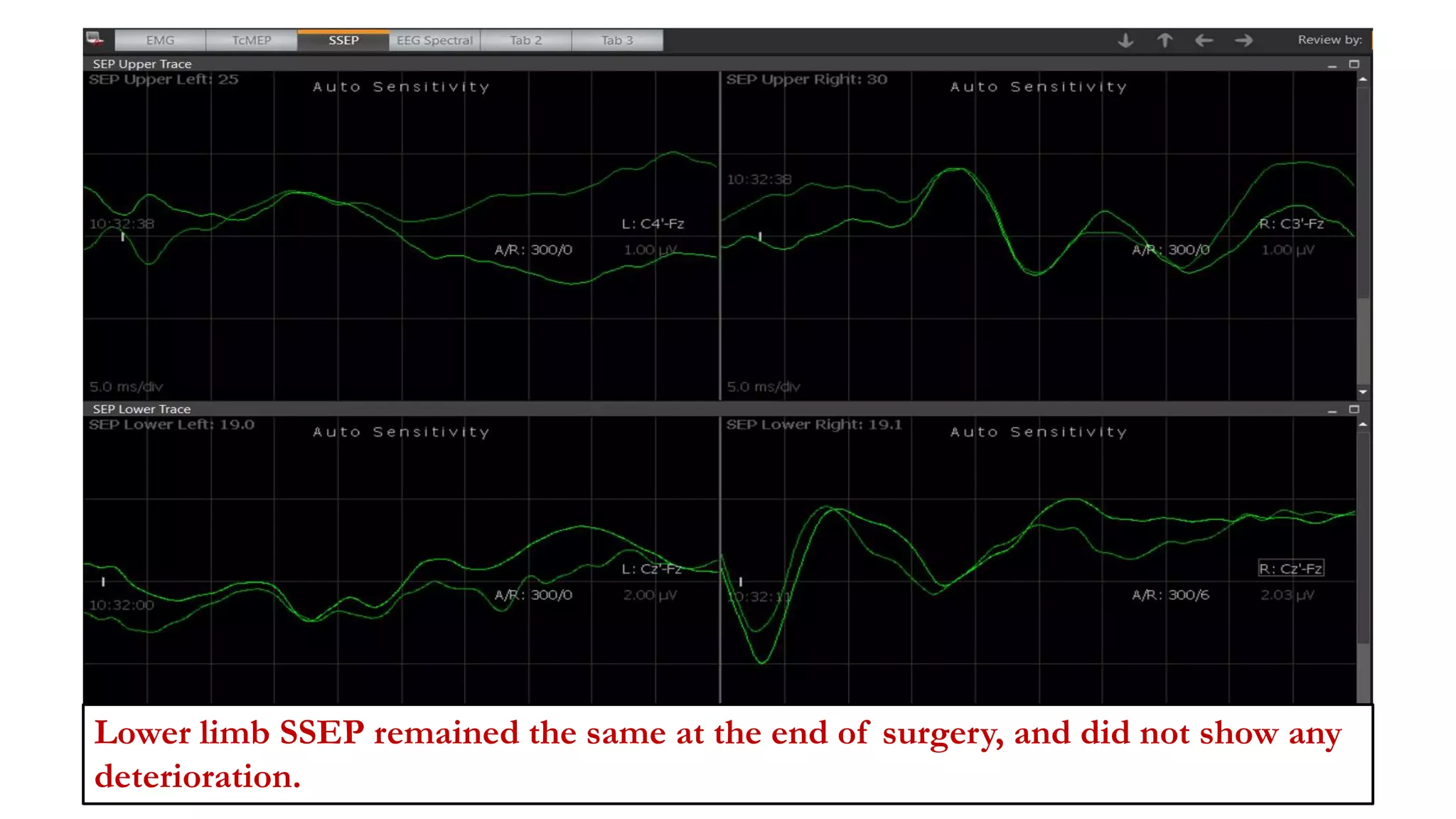
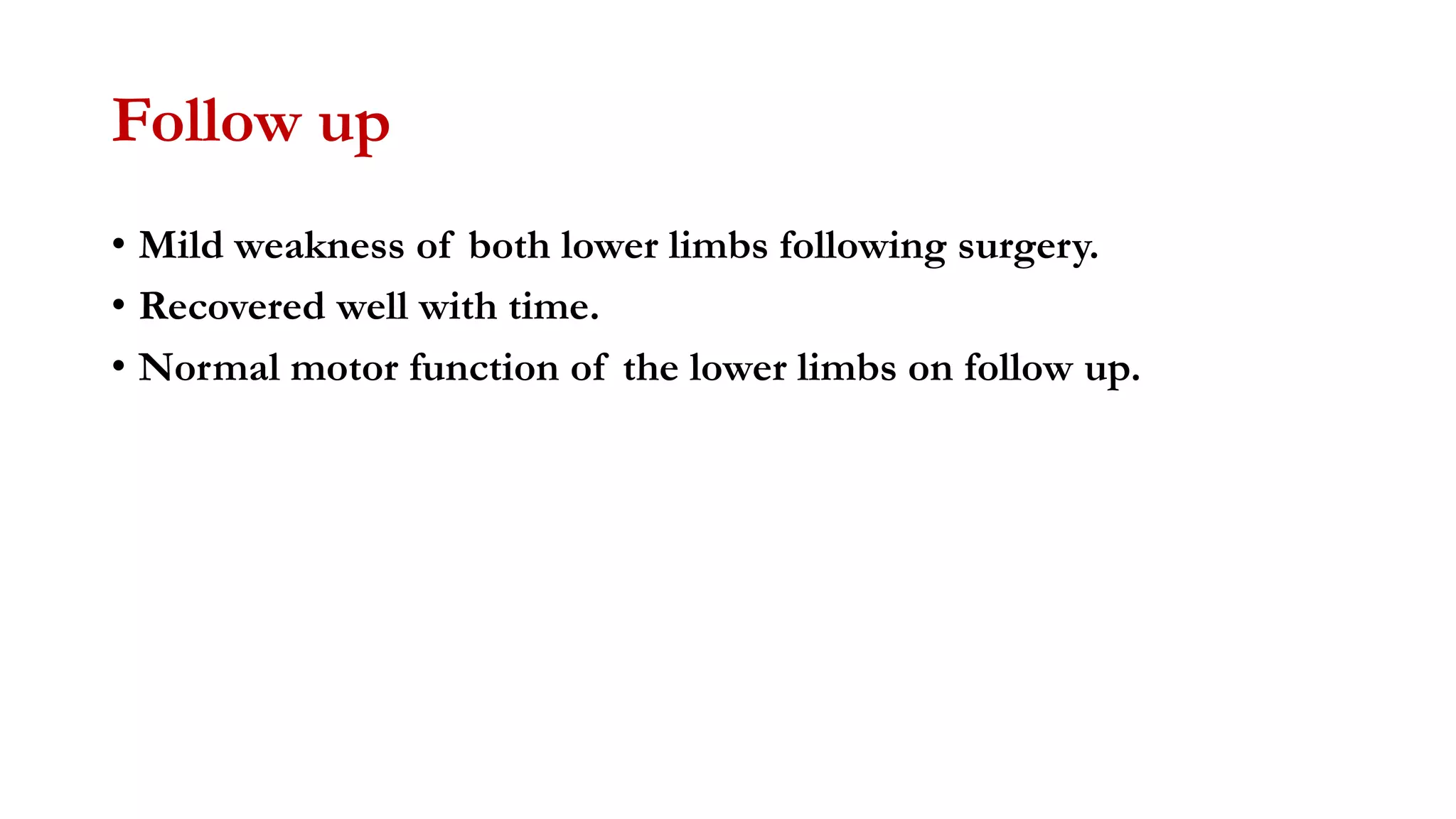
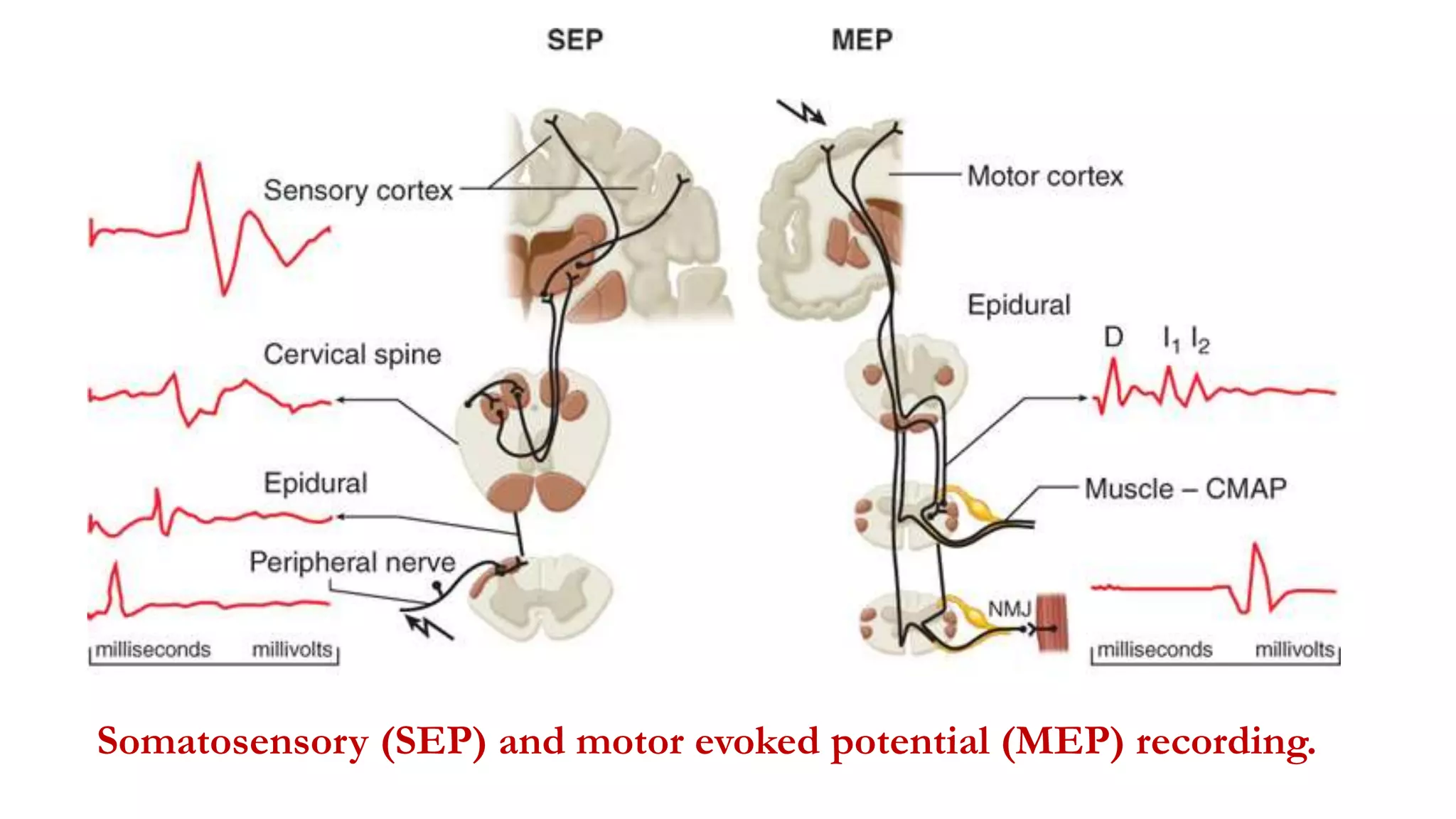
The document discusses intraoperative evoked potential monitoring, detailing various types of potentials such as somatosensory (SSEP), auditory (AEP), motor (MEP), and visual (VEP) for assessing anesthetized patients during surgery. It covers the physiological basis, stimulus techniques, recording methods, and clinical applications, emphasizing the importance of SSEPs in monitoring central somatosensory pathways during high-risk surgeries. It also highlights the limitations and potential complications associated with anesthetics and technical factors affecting monitoring accuracy.