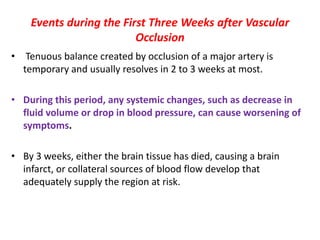
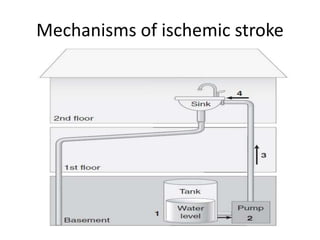
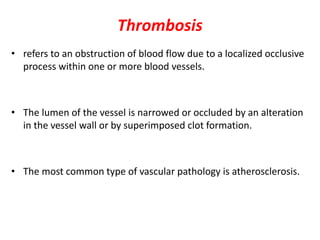
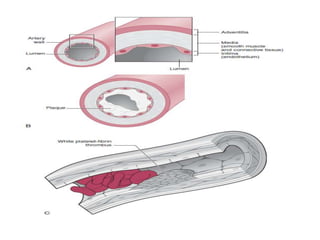
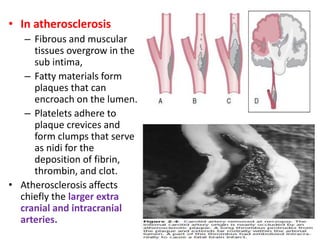
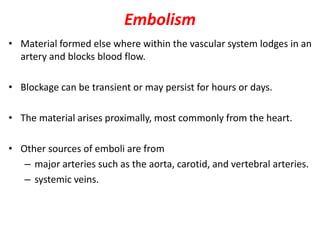
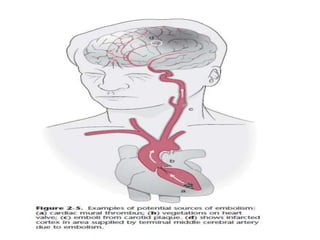
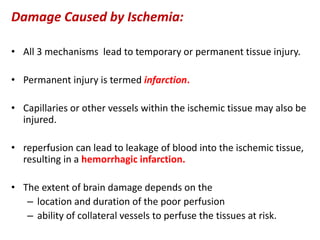
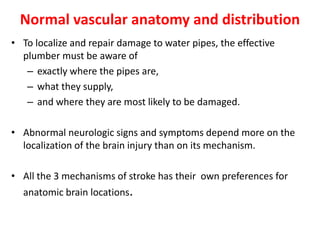
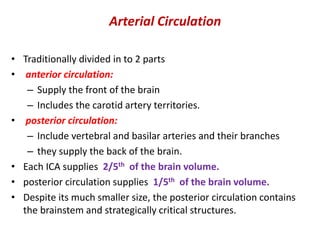
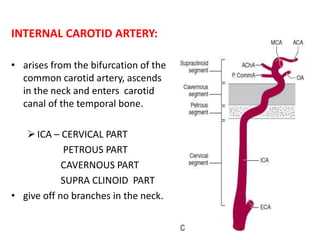
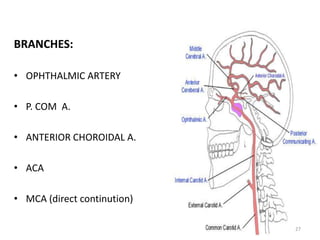
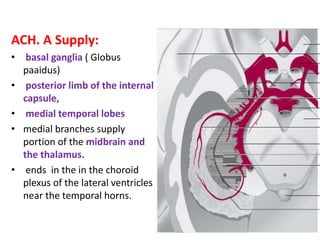
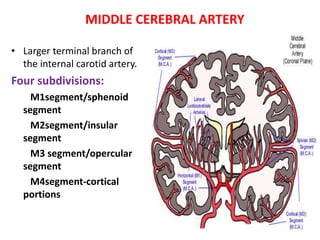
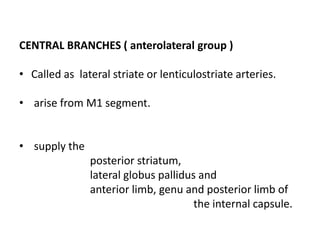
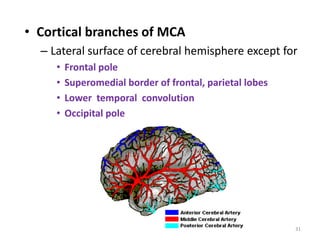
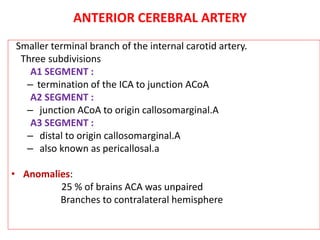
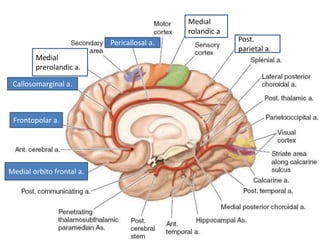
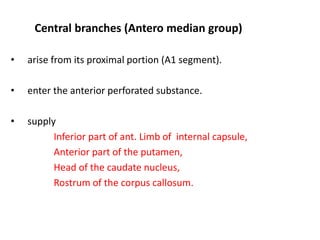
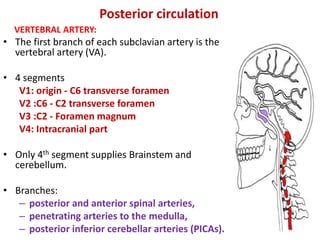
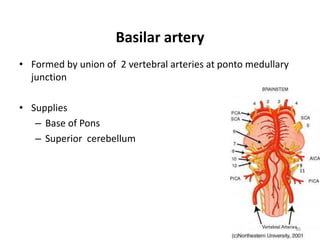
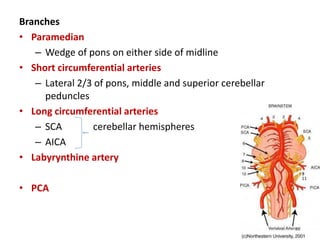
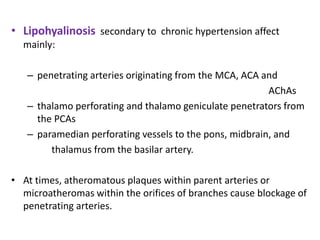
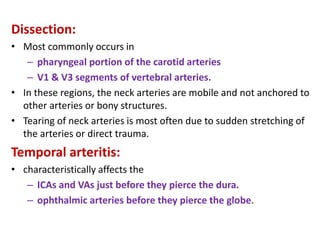
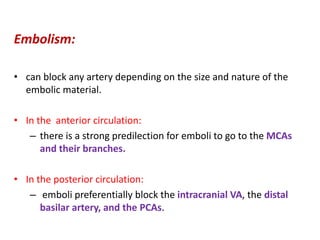
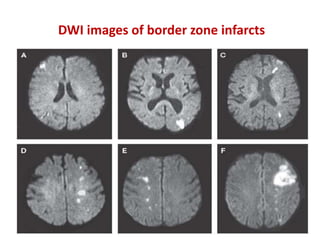
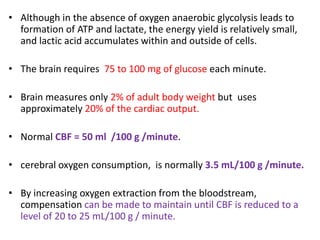
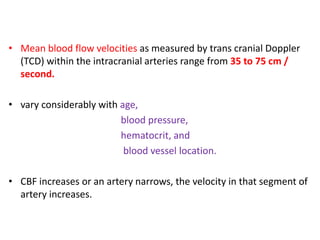
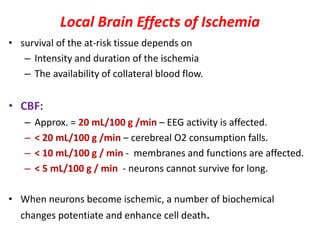
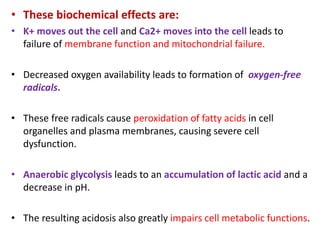
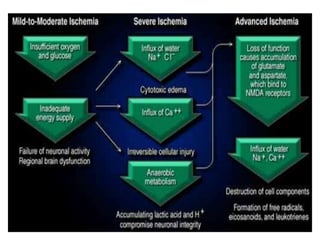
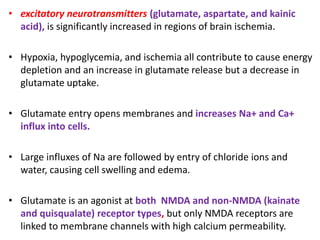
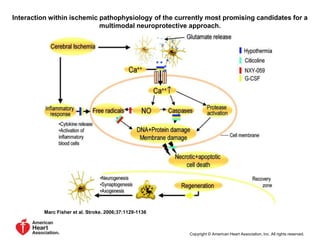
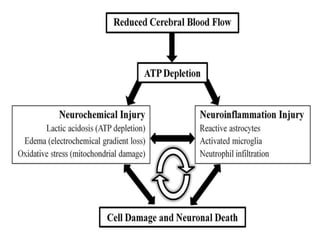
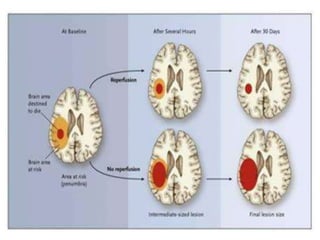
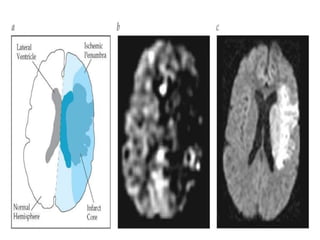
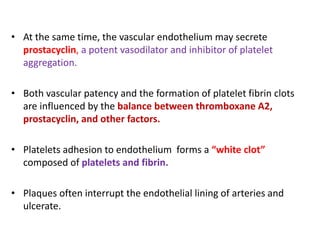
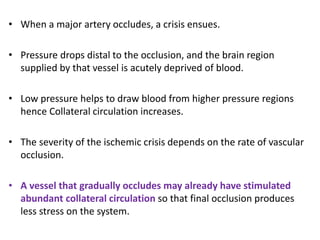
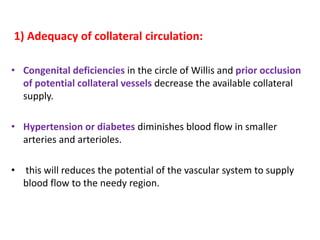
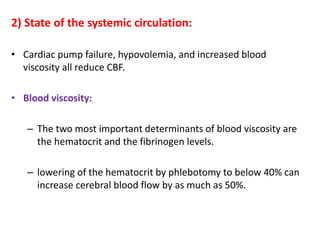
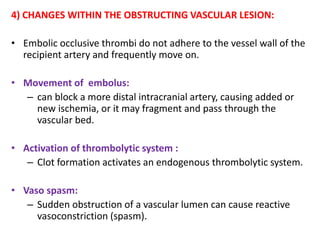
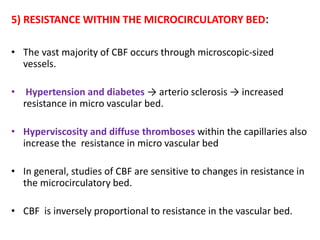
This document discusses the mechanisms and pathophysiology of ischemic stroke. There are three main mechanisms of ischemic stroke: thrombosis, embolism, and decreased systemic perfusion. Thrombosis occurs due to localized blood vessel narrowing, embolism occurs when a clot forms elsewhere and blocks a vessel, and decreased perfusion is caused by low blood pressure. The brain requires constant blood flow and oxygen to function, and ischemic stroke causes damage when blood and oxygen are cut off from brain tissue. The location and duration of ischemia determines the severity of injury.







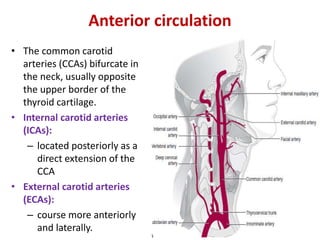
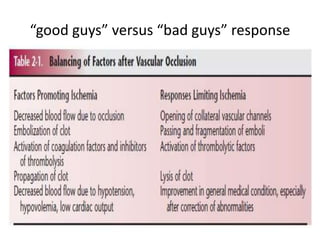
![External carotid artery [ECA]:
• supplies the face and major cranial
structures except for the brain.
• The 2 braches of ECAs channels can
act as collateral circulation if the ICAs
occlude
– The facial arteries:
• which course along the cheek
toward the nasal bridge.
– The preauricular arteries,
• which terminate as the
superficial temporal arteries.
• The internal maxillary artery and
ascending pharyngeal branches of the
ECAs also can contribute to collateral
circulation when an ICA occludes.](https://image.slidesharecdn.com/strokepathophysiology-190918100702/85/Stroke-patho-physiology-25-320.jpg)













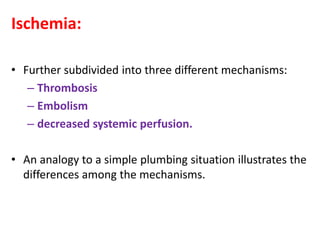
![(2) Vasogenic edema:
– fluid within the extracellular space.
– Also referred as wet edema because in such cases, the cut
surface of the brain oozes edema fluid.
– influenced by hydrostatic pressure factors and by osmotic
factors.
– breakdown of the blood-brain barrier → proteins and other
macromolecules enter the extracellular space → exert an
osmotic gradient → pulling water into the extracellular space.
– Preferentially involves cerebral white matter [d/t the
difference in compliance between gray and white matter].](https://image.slidesharecdn.com/strokepathophysiology-190918100702/85/Stroke-patho-physiology-76-320.jpg)