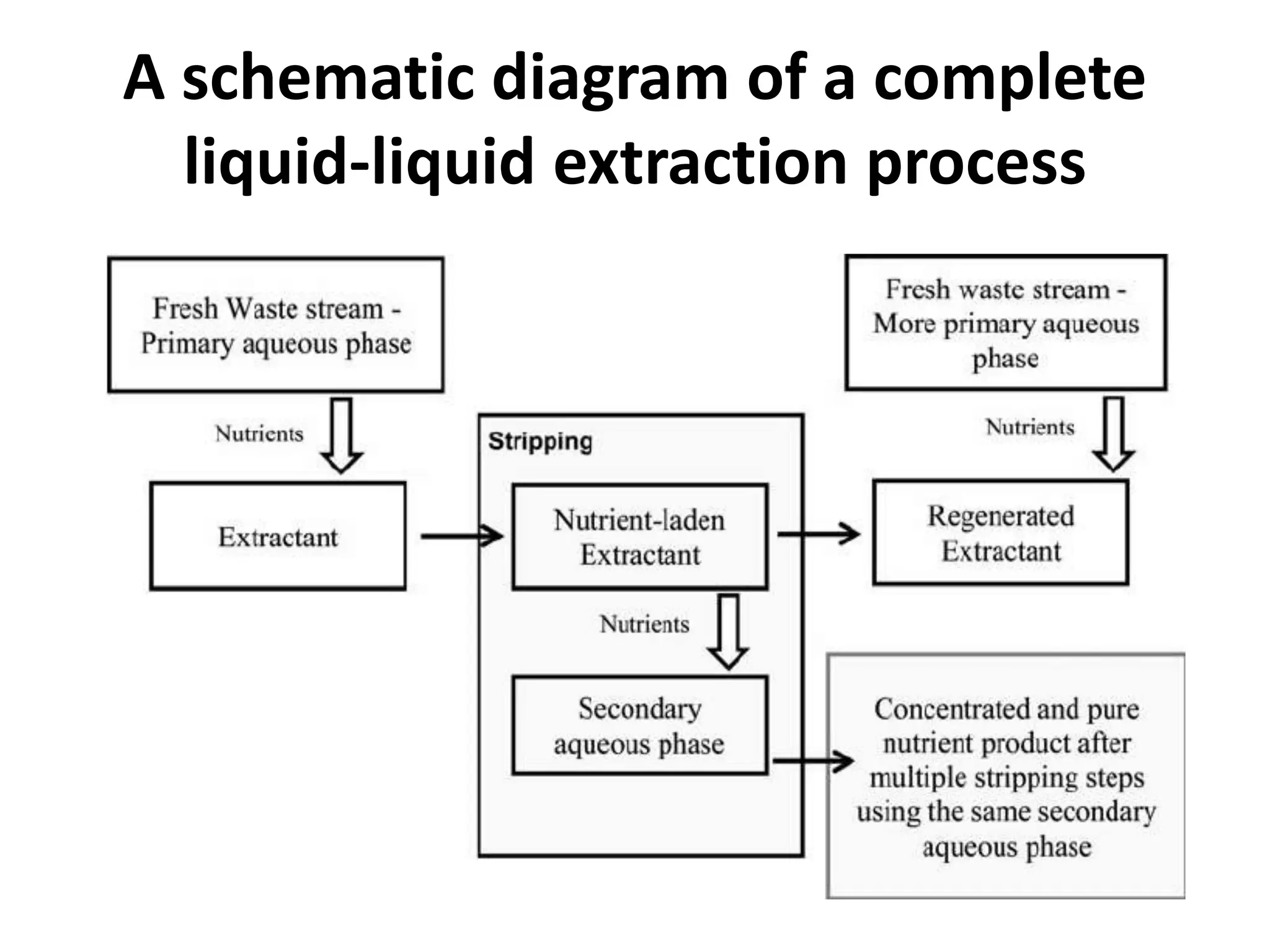
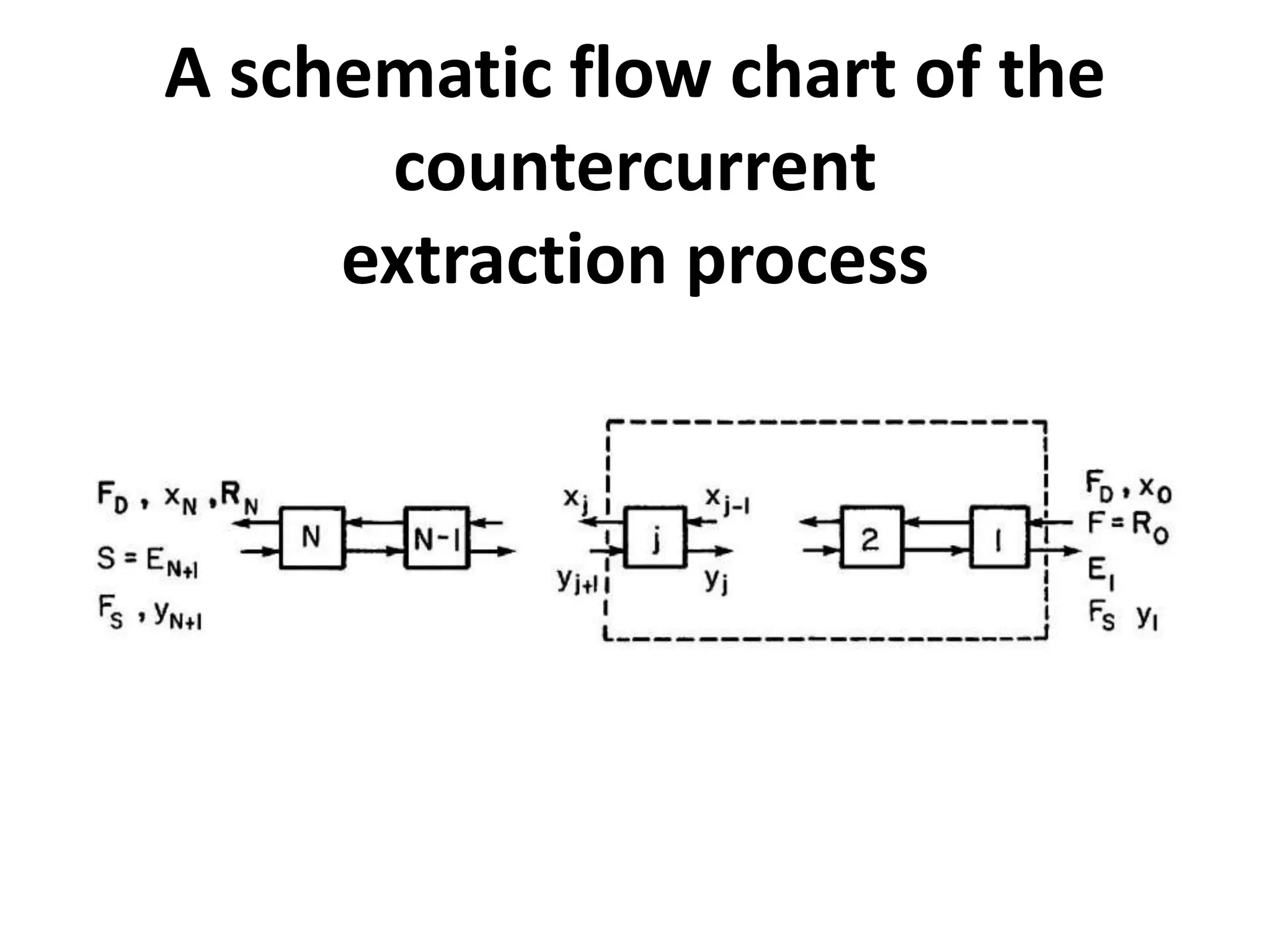
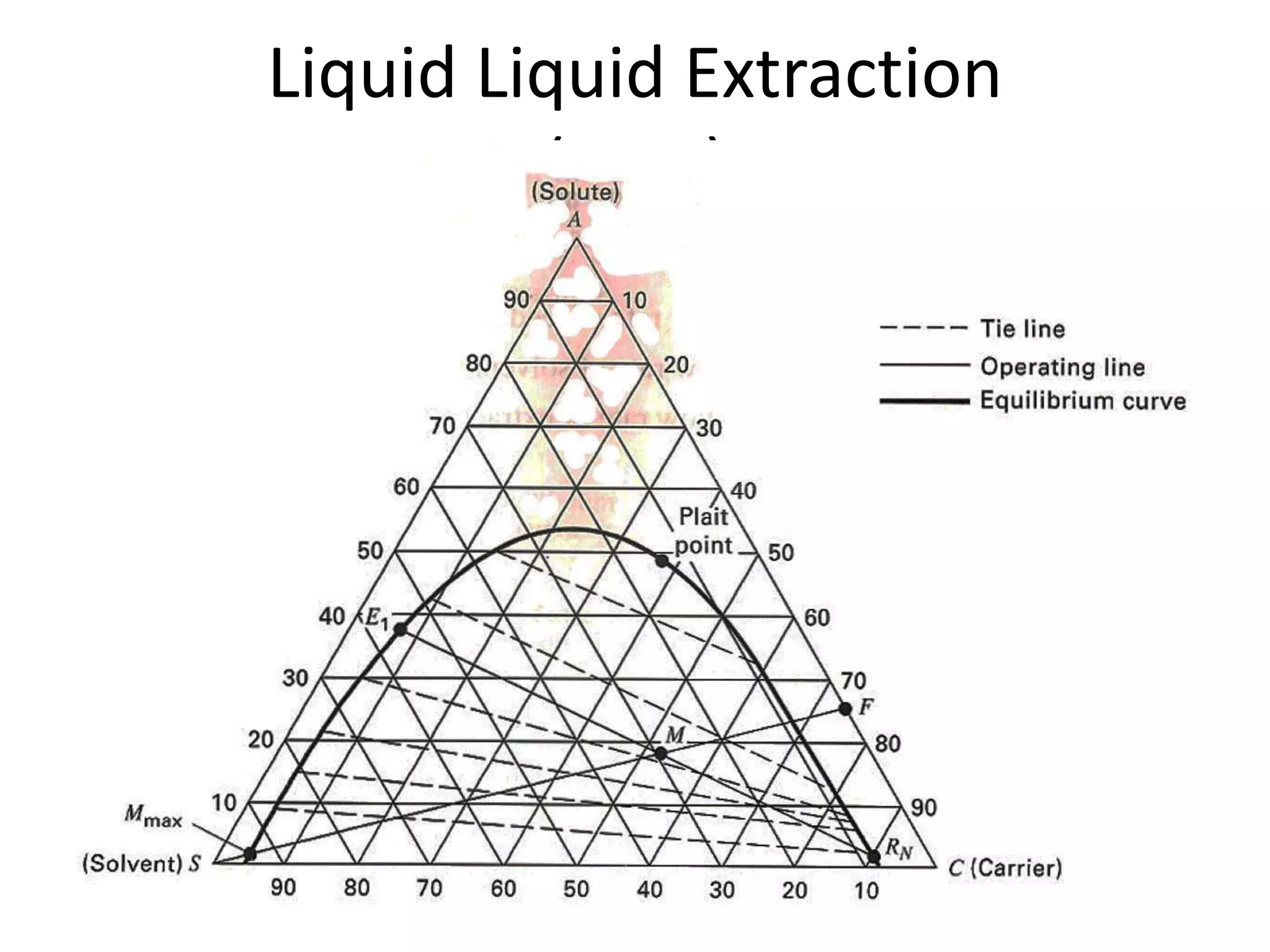
This document discusses liquid-liquid extraction in the food industry. It defines liquid-liquid extraction as a process that removes solutes from one liquid phase into another, without vaporization. Common examples given are separating penicillin from broth and aromatic hydrocarbons from paraffins. A diagram shows a countercurrent liquid-liquid extraction process, where feed and solvents are introduced on opposite sides and exit with different solute concentrations. Key factors in solvent selection are its ability to dissolve the desired solute more than the diluent, being immiscible with the diluent, and having other desirable properties. Types of extraction equipment and determining equilibrium stages are also briefly covered.