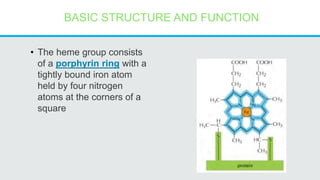
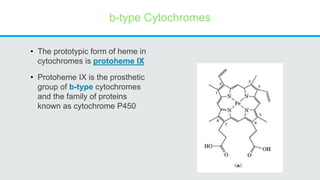
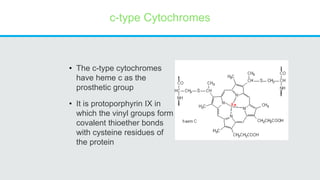
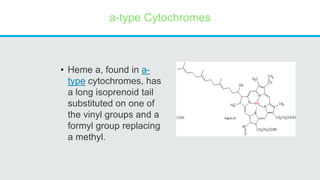
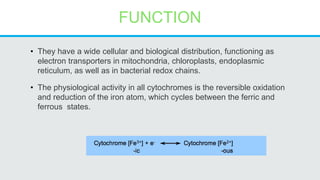
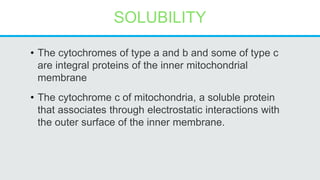
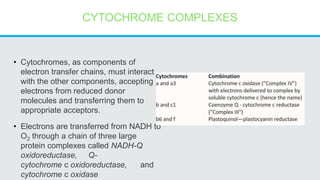
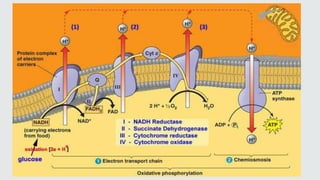
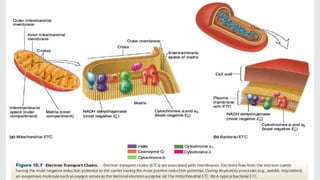
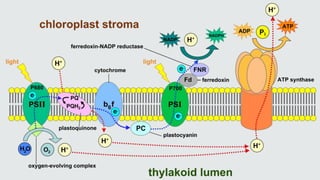
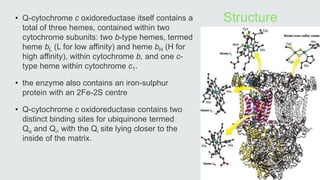
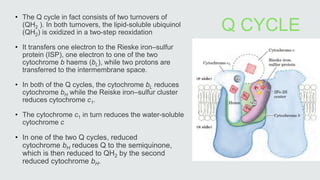
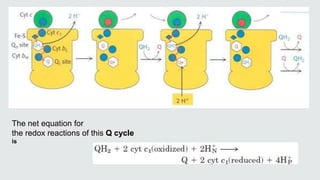
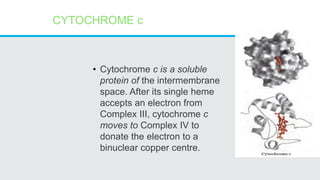
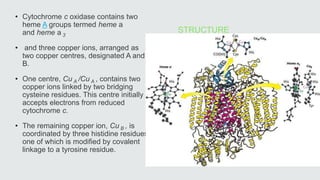
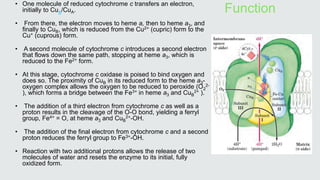
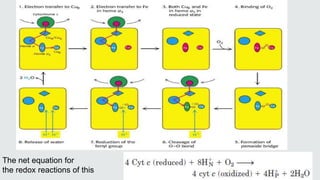
Cytochromes are proteins that contain heme groups and facilitate electron transfer in respiration and photosynthesis. They come in different types (a, b, c) that are distinguished by their prosthetic groups and absorption spectra. Cytochromes are components of electron transfer chains in the mitochondria and chloroplasts. They interact with other complexes like cytochrome bc1 and cytochrome c oxidase to transfer electrons from donors like NADH to final acceptors like oxygen. This facilitates ATP synthesis via the proton gradient generated across membranes.