The document summarizes Arrhenius theory of acids and bases as proposed by Svante Arrhenius in 1887. The key points are:
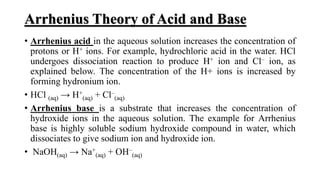
1) Arrhenius proposed that acids donate H+ ions and bases donate OH- ions when dissolved in water.
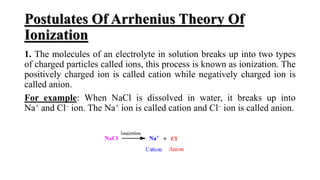
2) Electrolytes conduct electricity in solution by dissociating into ions, while nonelectrolytes do not dissociate or conduct.
3) The theory explained several phenomena like heat of neutralization and differentiation of strong/weak electrolytes but had limitations like not explaining ion formation mechanisms.