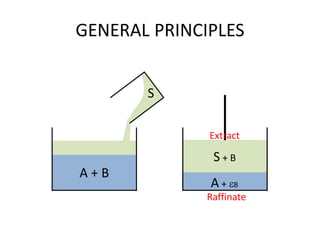
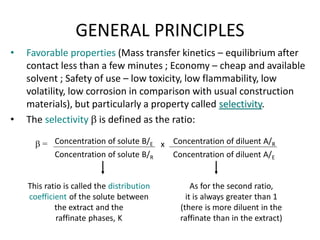
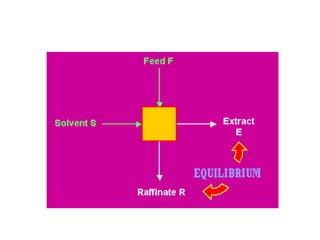
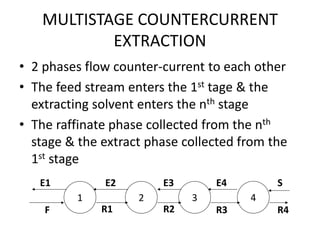
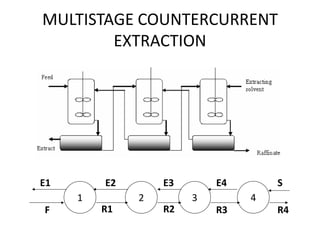
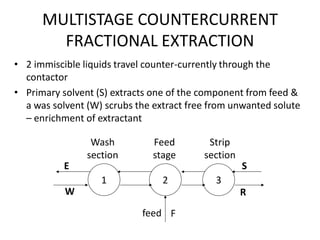
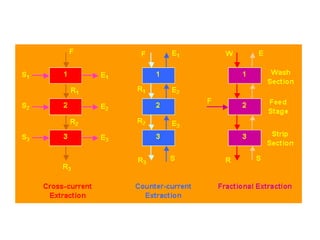
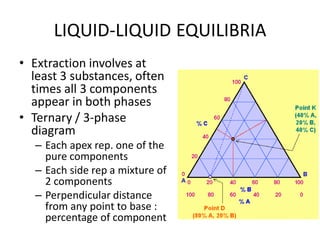
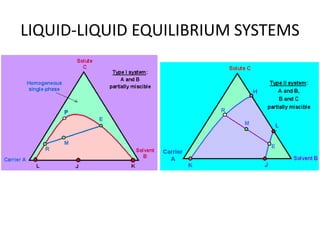
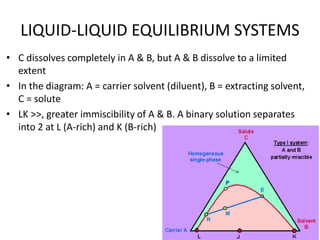
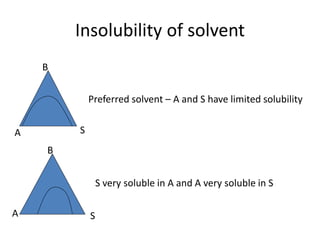
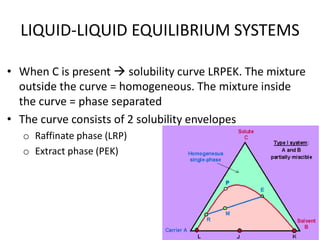
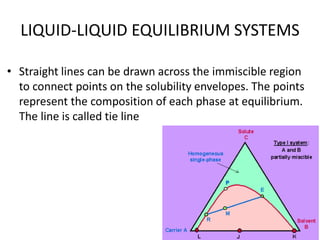
This document discusses liquid-liquid extraction (LLE), a process that typically involves a reaction step followed by various separation techniques, including distillation and solvent extraction, when distillation is unsuitable. It explains the principles of extraction, including the roles of feed, solvent, raffinate, and extract phases, highlighting the process's advantages such as lower cost and safety compared to traditional methods. Multiple extraction techniques, mass balances, and phase compositions for both single-stage and multistage extractions are explored to illustrate the extraction process in detail.





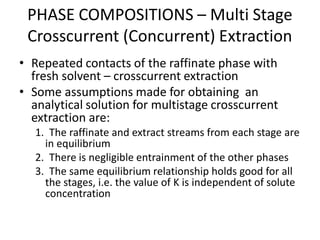
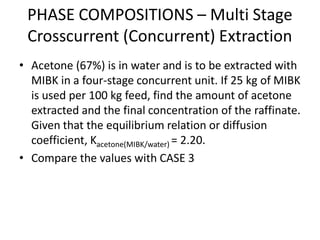
![PHASE COMPOSITIONS – Multi Stage
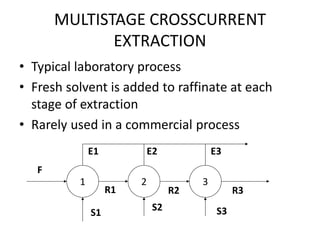
Crosscurrent (Concurrent) Extraction
• The calculation is the repeat of that for single stage extraction. The
amount of liquid can be different in each stage
• Consider solute C in carrier A, mixed with solvent B. For
crosscurrent (concurrent) contact with immiscible solvents a simple
mass balance for solute C at steady state gives the operating line:
• xn = [A/(A+Bm)]n xf
where xn = kg C/kg A in raffinate
A = mass of carrier A in feed
B = mass of solvent B added
m = distribution coefficient
xf = kg C/kg A in feed](https://image.slidesharecdn.com/liquidextraction1-250119144229-860b2f10/85/topic-liquid-liquid-extraction-process-pptx-29-320.jpg)
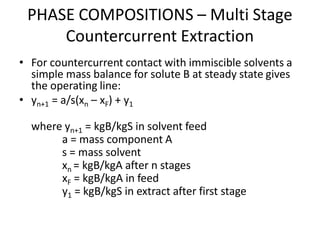
![PHASE COMPOSITIONS – Multi Stage
Crosscurrent (Concurrent) Extraction
• Compound A (10%) is in toluene and is to be extracted
with water in a five-stage concurrent unit. If 25 kg of water
is used per 100 kg feed, find the amount of Compound A
extracted and the final concentration of the raffinate.
Given that the equilibrium relation or diffusion coefficient,
KCompound A(water/toluene) = 2.20.
• xn = [A/(A+Bm)]n xf
where xn = kg C/kg A in raffinate
A = mass of carrier A in feed
B = mass of solvent B added
m = distribution coefficient
xf = kg C/kg A in feed](https://image.slidesharecdn.com/liquidextraction1-250119144229-860b2f10/85/topic-liquid-liquid-extraction-process-pptx-30-320.jpg)