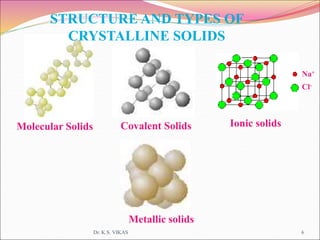
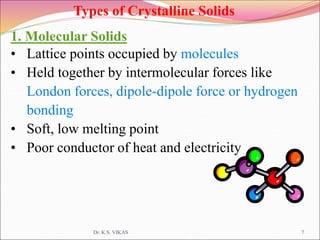
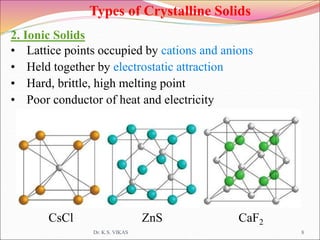
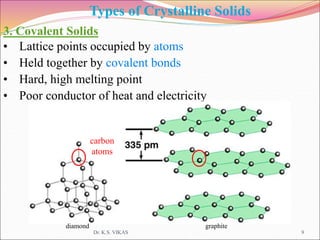
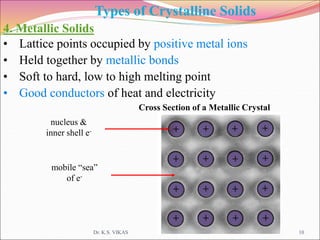
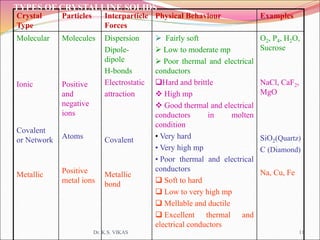
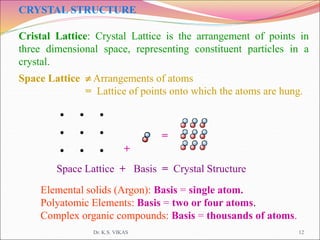
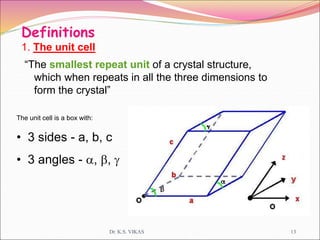
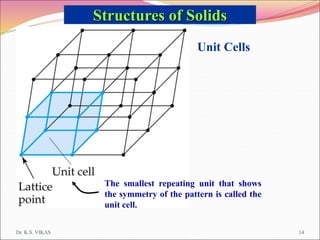
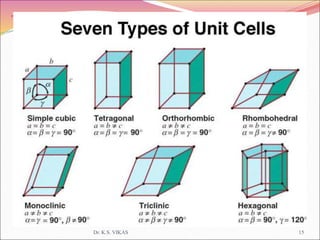
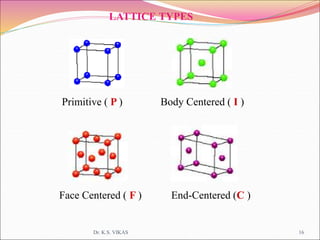
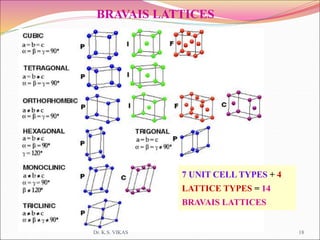
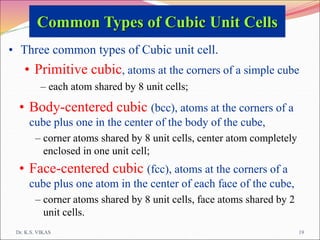
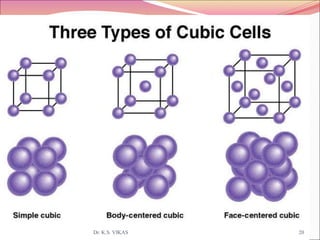
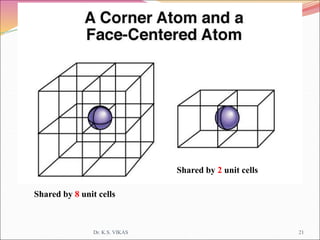
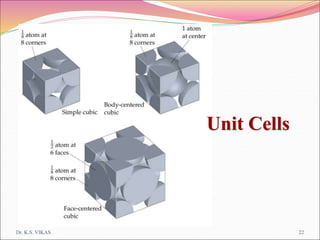
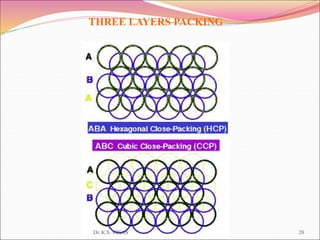
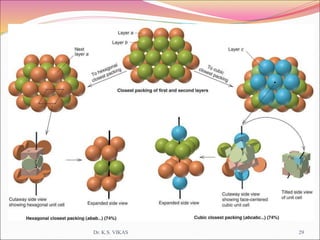
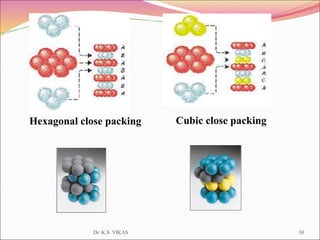
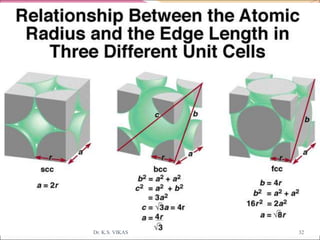
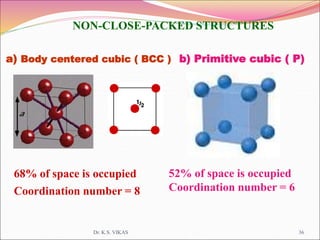
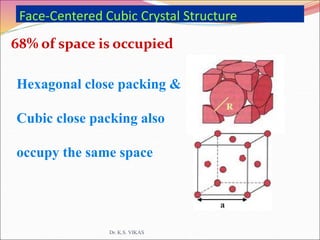
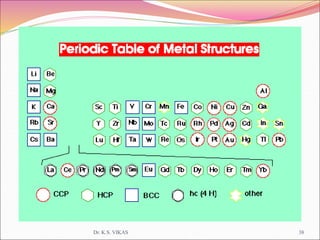
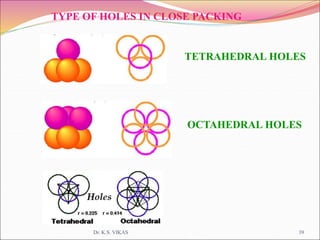
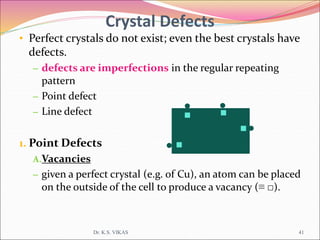
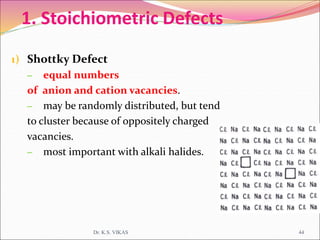
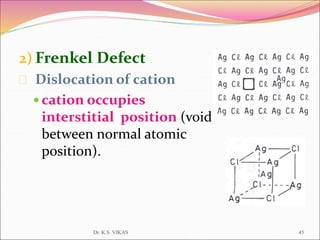
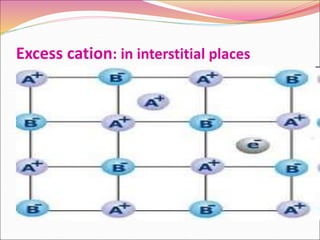
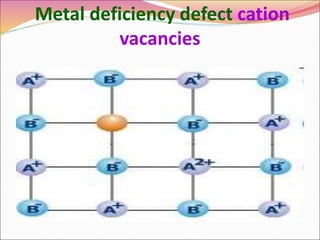
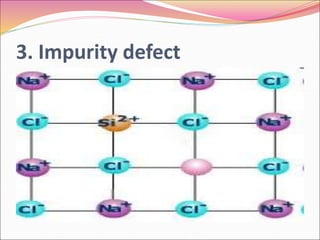
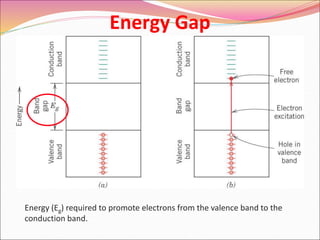
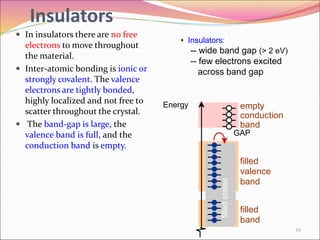
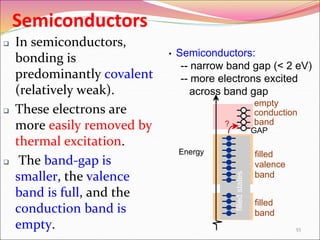
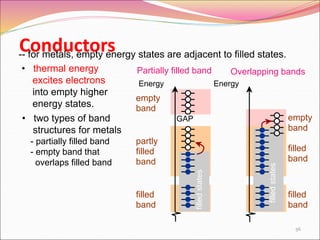
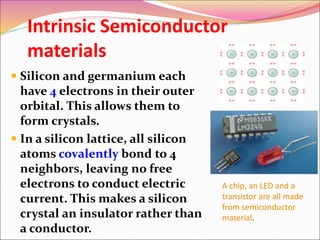
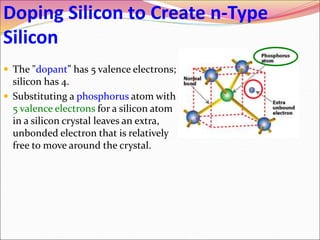
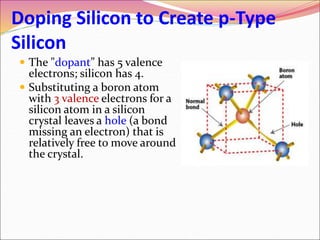
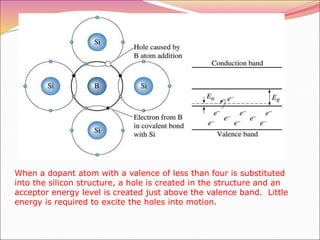
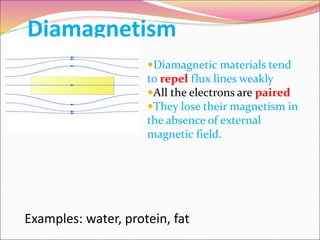
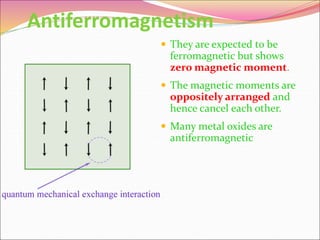
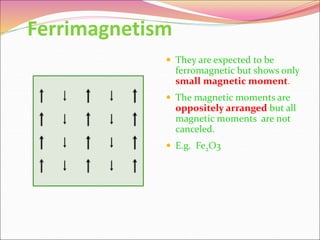
This document discusses different types of solids and their structures. It describes two main types of solids - crystalline and amorphous. Crystalline solids have long-range order while amorphous solids have short-range order. Crystalline solids are further divided into molecular, ionic, covalent and metallic solids based on the type of bonding between particles. The document also discusses crystal structures, unit cells, Bravais lattices and close packing of spheres. It covers defects in solids and electrical, magnetic and optical properties of materials.