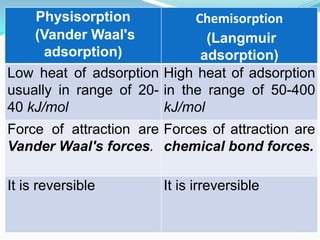
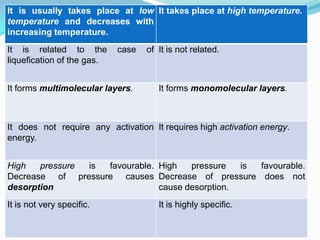
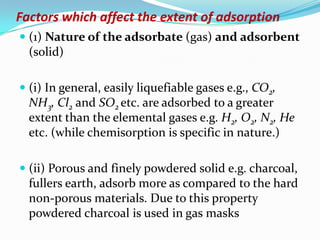
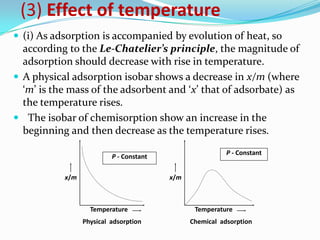
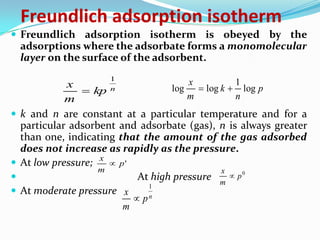
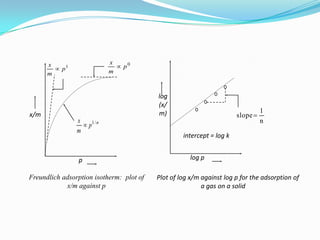
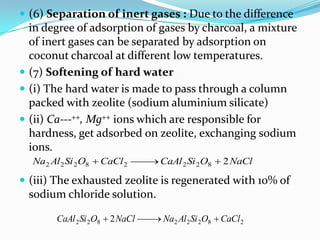
The document discusses surface chemistry and adsorption. It defines adsorption as molecules of a substance accumulating on the surface of a solid or liquid. Adsorption occurs due to unbalanced surface forces and is exemplified by ammonia adsorbing onto charcoal. Adsorption can be physical or chemical depending on the strength of attraction. Factors like temperature, surface area, and gas/solid properties affect adsorption extent. Adsorption finds applications in areas like vacuum production, gas masks, desiccation, catalysis and water softening.