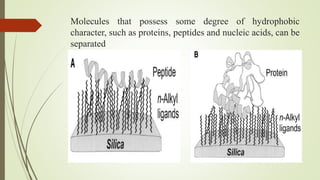
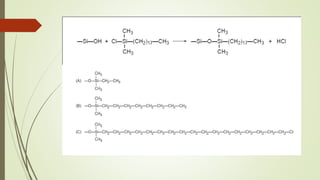
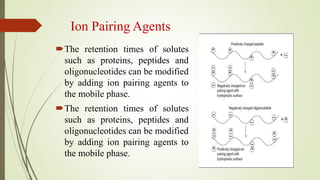
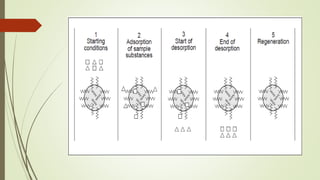
Reversed phase chromatography is an adsorption technique used to separate nonpolar substances. It works by having a nonpolar stationary phase and a polar mobile phase, opposite of normal phase chromatography. Molecules like proteins, peptides, and nucleic acids can be separated using reversed phase chromatography. The separation depends on the hydrophobic binding of solutes from the mobile phase to the hydrophobic ligands attached to the stationary phase. Common stationary phases use silica beads with attached alkyl hydrocarbon chains of varying lengths. Gradient elution with mixtures of water and organic solvents like acetonitrile or methanol is typically used for separation. Reversed phase chromatography has applications in preparative purification of proteins, peptides, and other biomolecules.