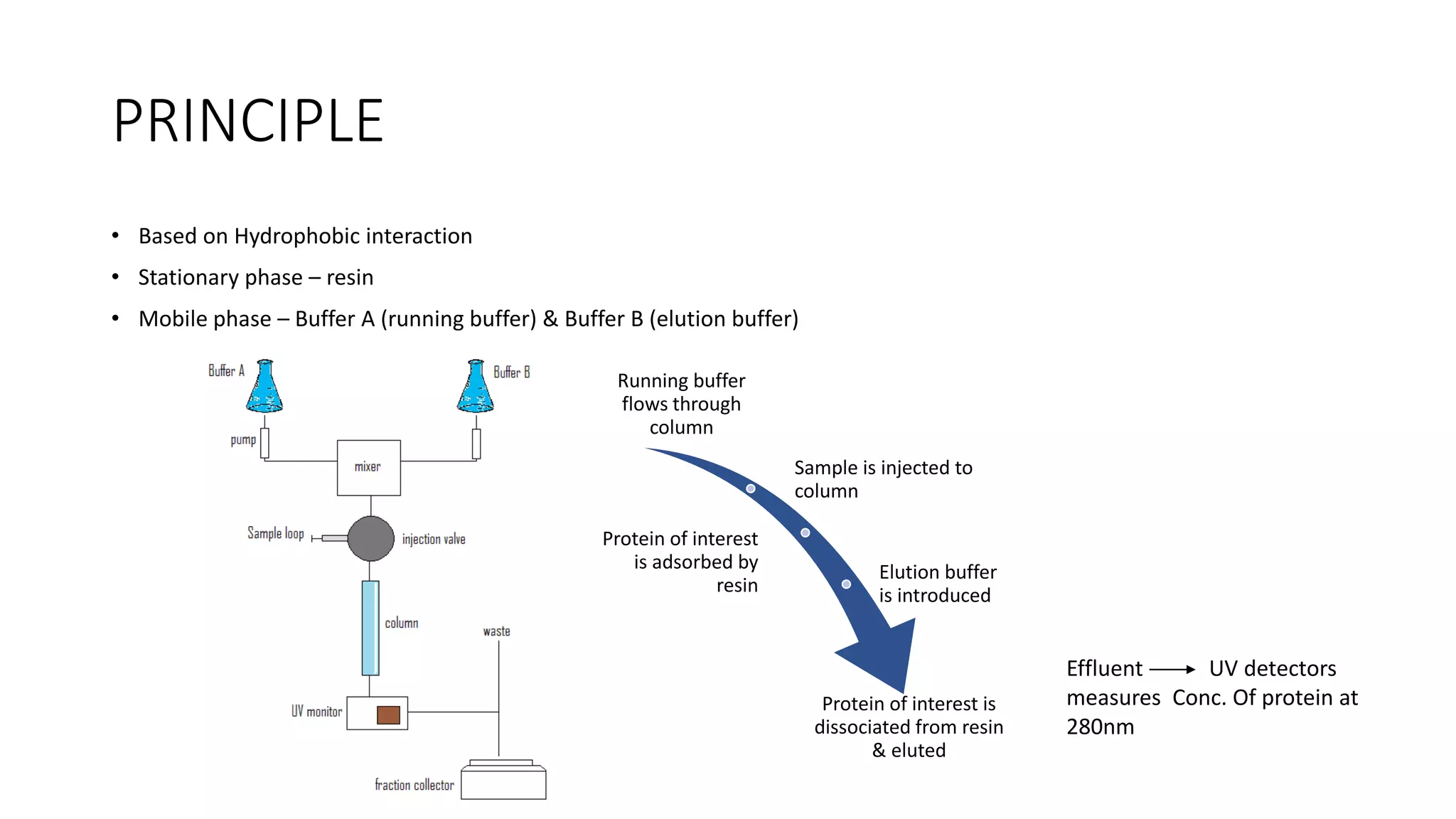
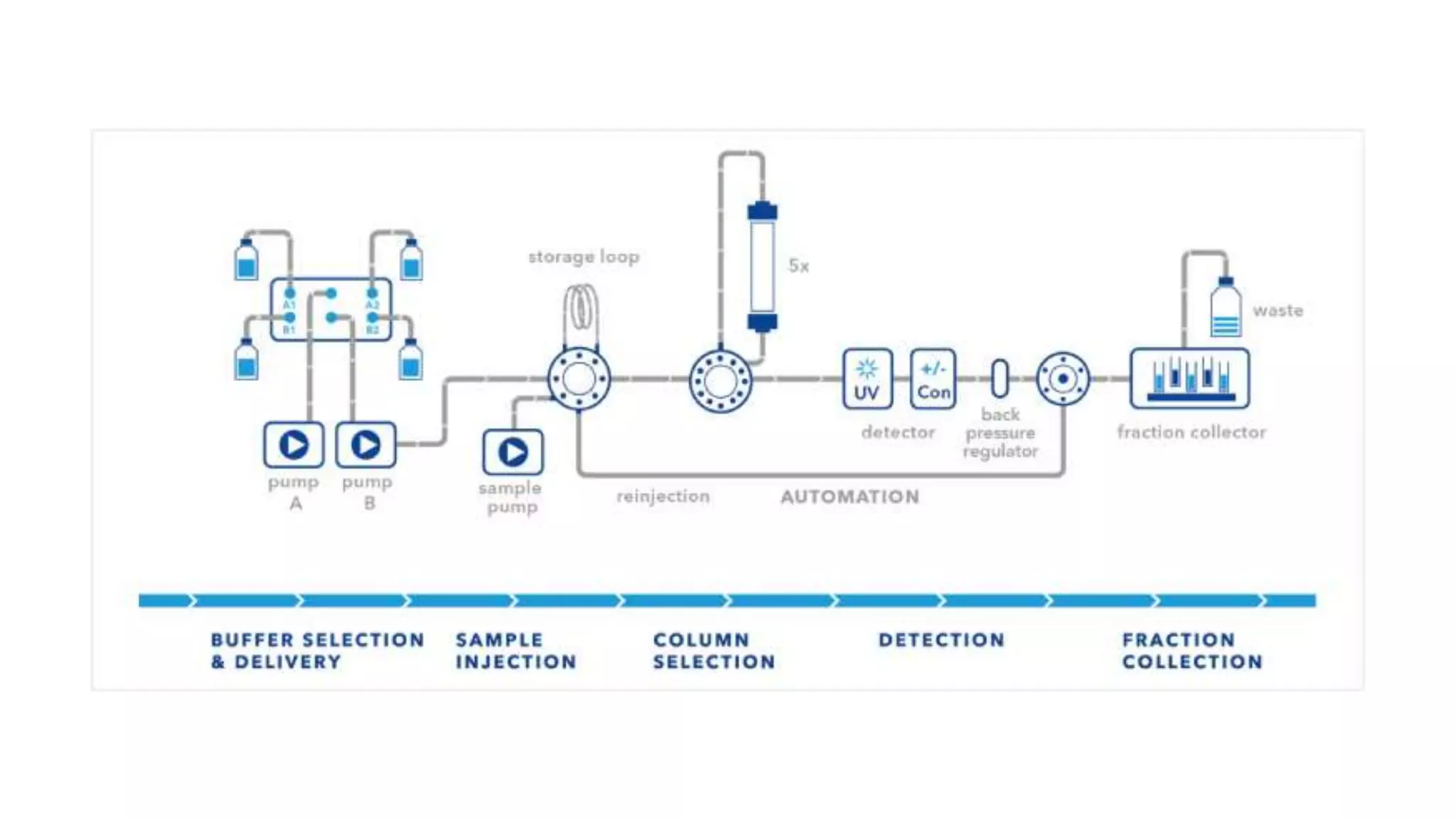
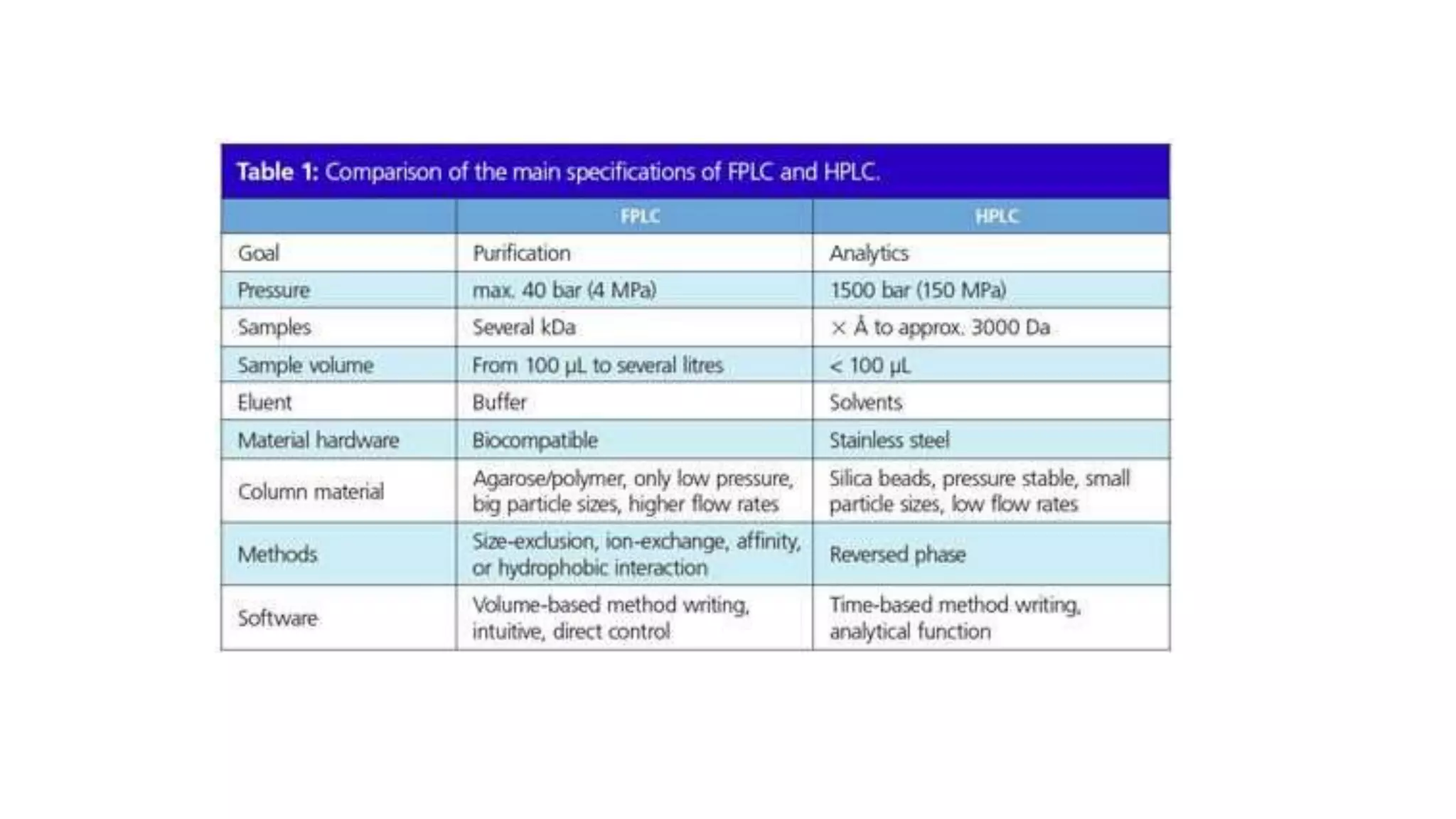
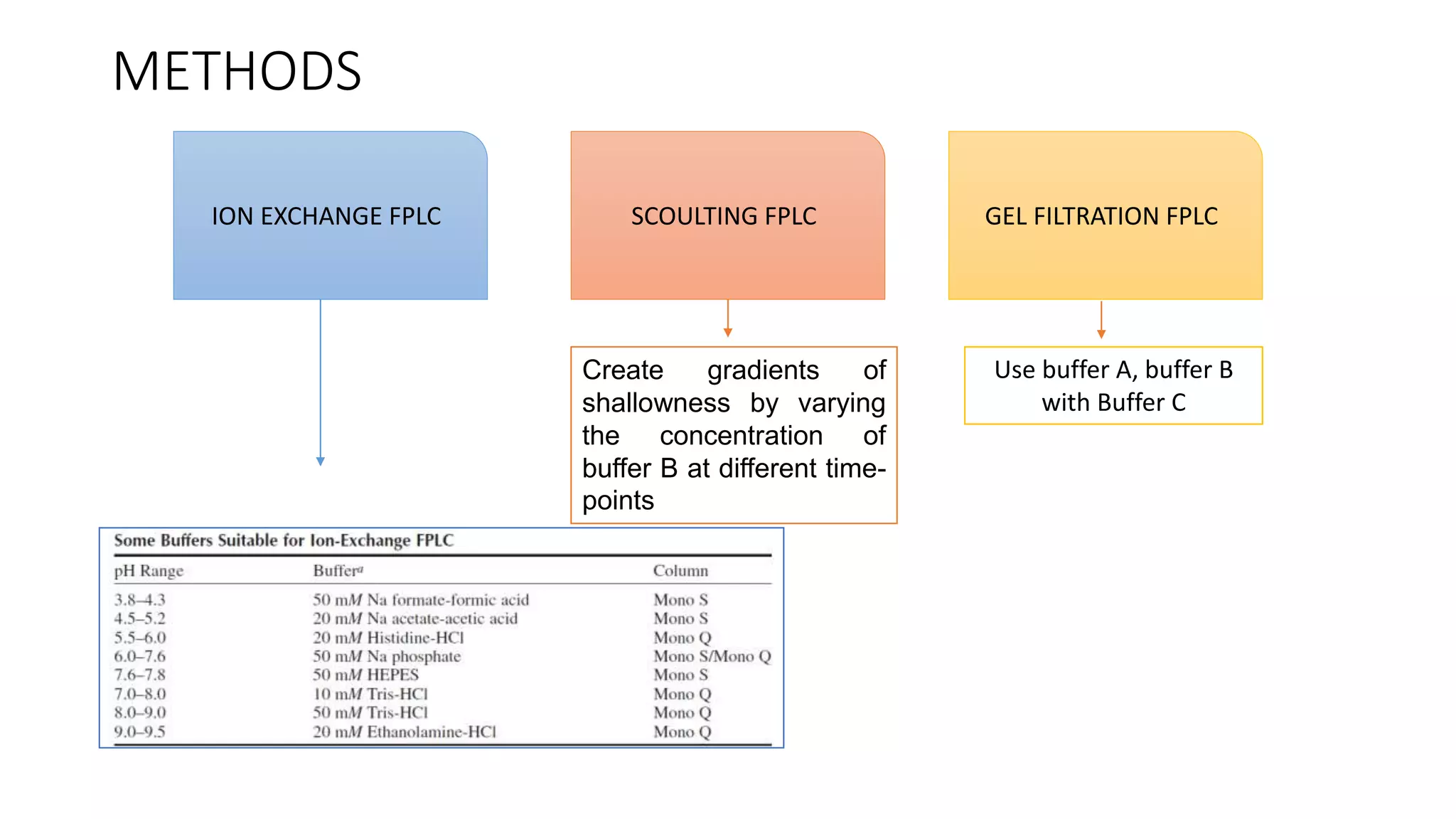
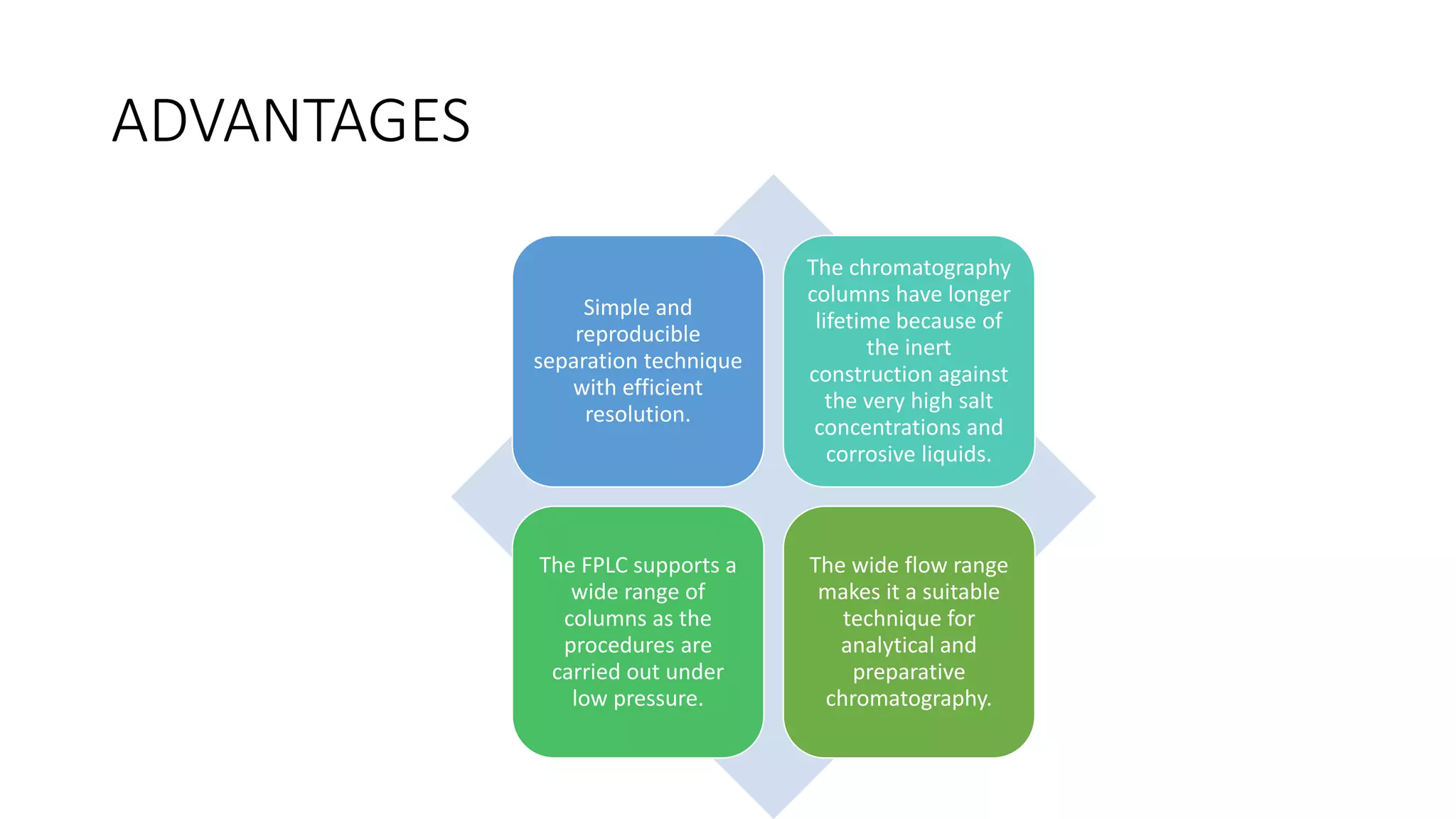
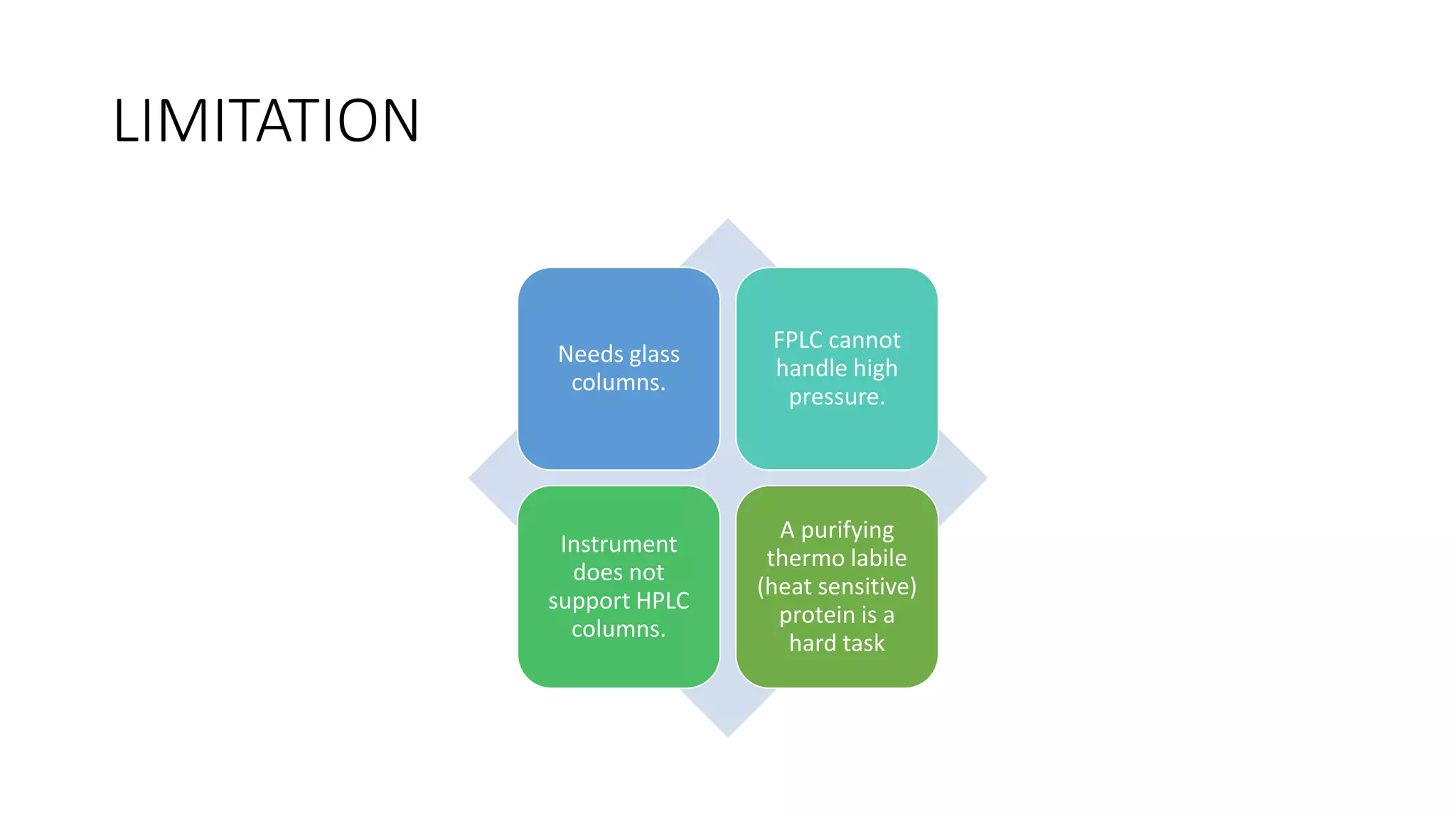
Fast protein liquid chromatography (FPLC) is a type of liquid chromatography used to analyze or purify proteins. It introduces samples onto a column containing resin beads, then uses buffers to differentially elute bound protein. FPLC allows separation of heat-labile biomolecules like proteins under mild conditions like 4°C. It has advantages like simple reproducible separation, efficient resolution, and support for a wide range of columns and procedures under low pressure. Limitations include needing glass columns and inability to handle high pressures.