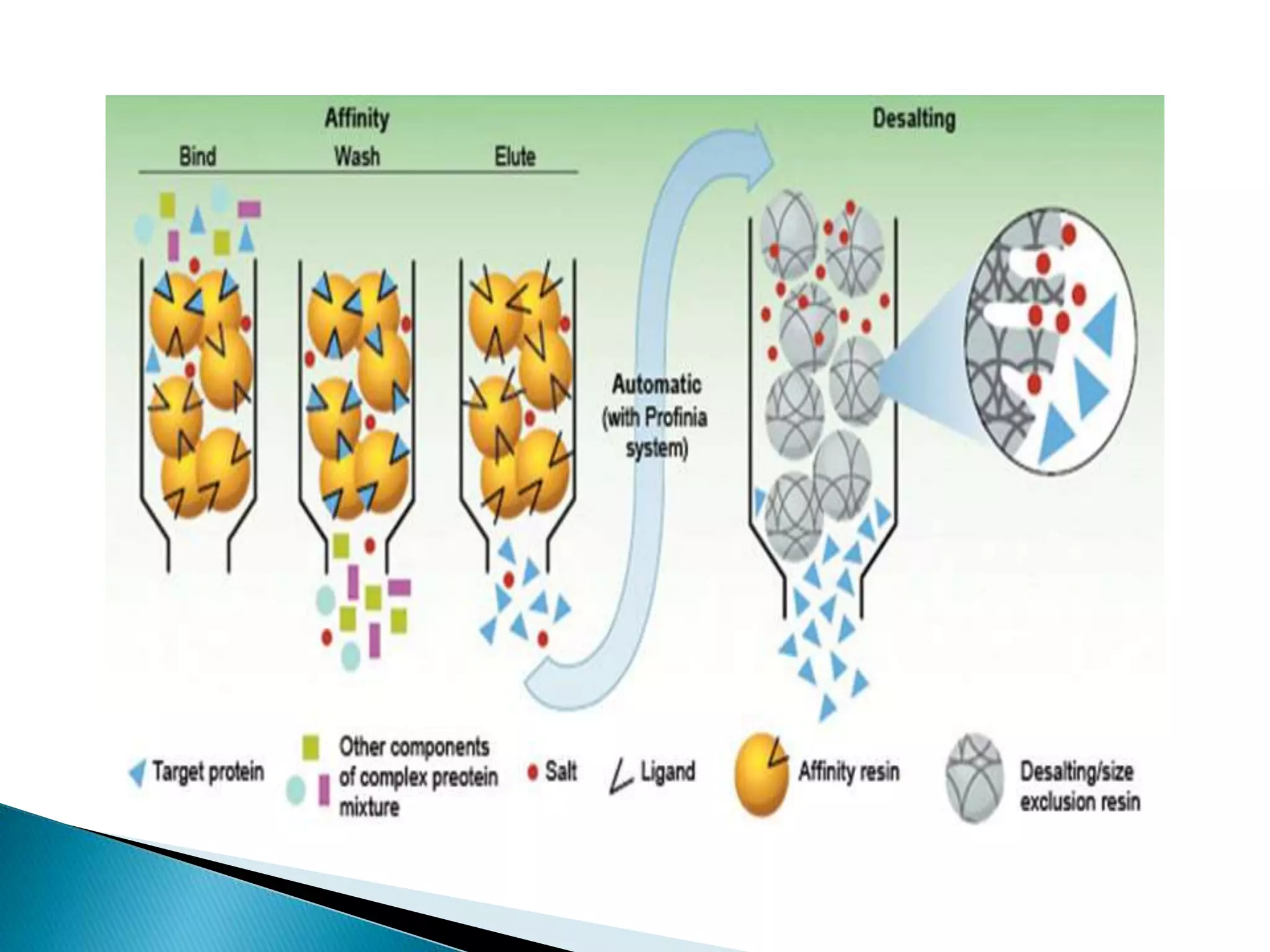
Affinity chromatography is a type of liquid chromatography that uses reversible biological interactions or molecular recognition to separate sample components. It works by attaching a ligand with affinity for the target molecule to a stationary phase. When a sample mixture passes through the column, only molecules that bind to the ligand will be retained on the stationary phase while non-binding molecules pass through. Retained molecules can then be eluted by altering conditions to break the binding interaction selectively. Affinity chromatography is useful for purifying and studying enzymes, proteins, and other biomolecules due to its high specificity and ability to achieve a high degree of purity.