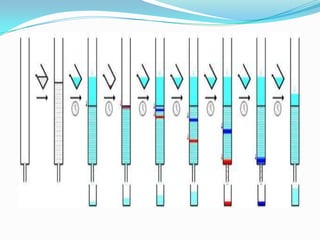
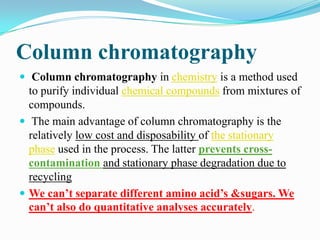
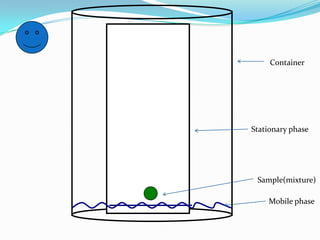
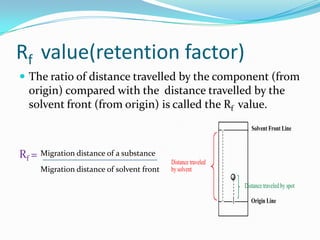
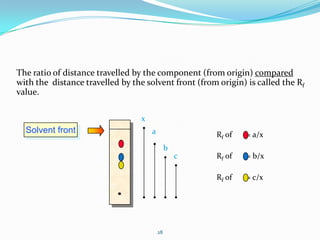
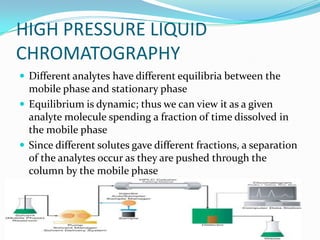
Chromatography is a technique used to separate mixtures by exploiting differences in how components interact with stationary and mobile phases. It was discovered in 1906 by Tswett and is commonly used for separation, purification, identification, and quantification of mixture components. The key components are a stationary phase, mobile phase, and sample mixture. Different types of chromatography exist depending on the phase types, including solid-liquid, liquid-liquid, gas-liquid, and liquid-solid. Common applications include analyzing pharmaceuticals, detecting substances in blood/tissue, forensic analysis, and environmental monitoring.