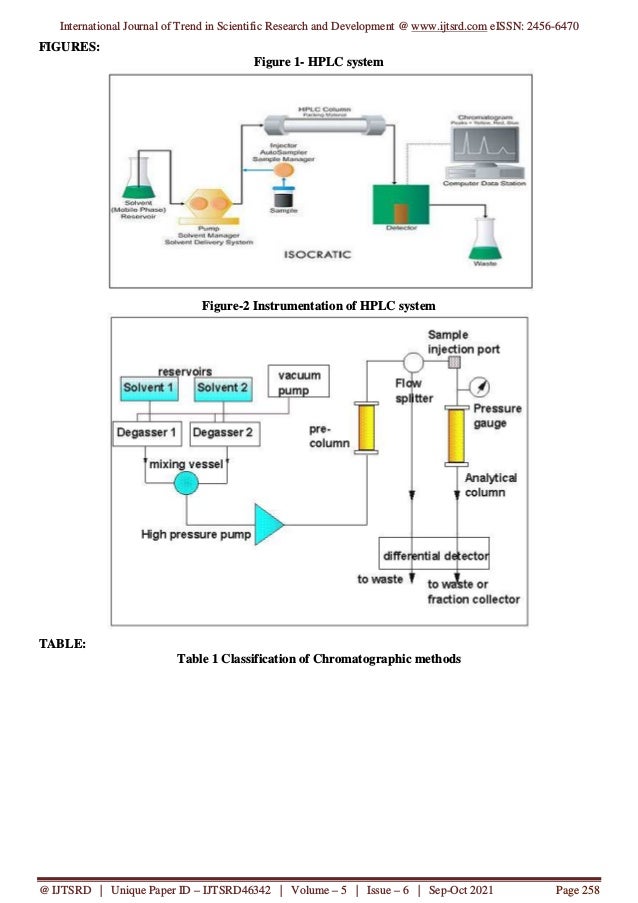
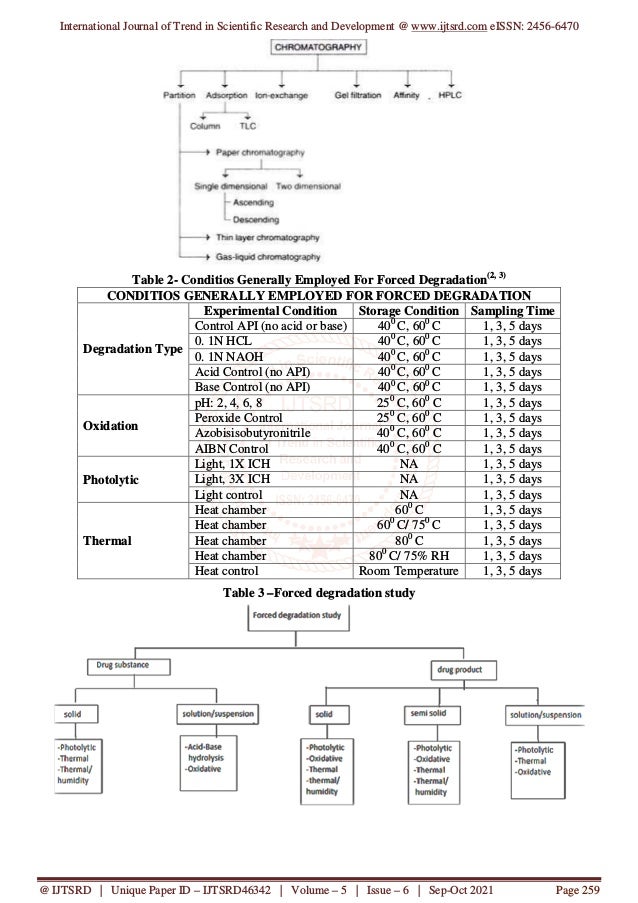
This document reviews the development of stability-indicating high-performance liquid chromatography (HPLC) methods, emphasizing their importance in analyzing drug stability by separating impurities formed during drug manufacturing. It discusses forced degradation studies which are crucial for understanding degradation pathways and ensuring the reliability of pharmaceutical products. Various chromatographic techniques and system components are detailed, including mobile phase selection, detector types, and column materials essential for improving analysis accuracy and efficiency in the pharmaceutical industry.






![International Journal of Trend in Scientific Research and Development @ www.ijtsrd.com eISSN: 2456-6470
@ IJTSRD | Unique Paper ID – IJTSRD46342 | Volume – 5 | Issue – 6 | Sep-Oct 2021 Page 256
in providing effective information about the
degradation products and degradation pathways that
could during storage.2
Q1A (R2) – Stability Testing of
New Drug Substances and Products, states: “Stress
testing is likely to be carried out on a single batch of
the drug substance. The testing should contains effect
of temperature (in 10°C increments (i.e., 50°C, 60°C)
above that for accelerated testing), humidity (i.e.,
75% relative humidity or greater) where appropriate
photolysis and oxidation of drug substance(2,26,27).
Appropriate timing
The stability testing of the drug substance should
assess stress studies in different pH solutions in the
presence of oxygen and light and at humadity level
and elevated temperature.
These studies are most effective if development in
phase 1 preclinical trial or preclinical development. A
forced degradation study on drug substance at this
stage will provide timely guidance for development in
manufacturing process, proper selection of stability-
indicating analytical techniques and ensure there is
enough time for degradation pathway interpretation,
degradation pathway identification and development
of stress condition.
Experimental Design
In designing forced degradation studies, it must be
remembered that more tough condition than those
used for accelerated studies (25°C/60% RH or
40°C/75% RH) should be used.
The forced degradation condition should be followed-
1. Acid and base hydrolysis,
2. Hydrolysis at various ph,
3. Thermal degradation,
4. Photolysis, and
5. Oxidation.(2,27)
Initially the experiment should be focused on
determining the condition that degrade the drug
approximately 10%. The condition should be
summarized below in table 2.
CONCLUSION
Stability-indicating method is an analytical procedure
which is used to check purity of drug sample or drug
substance. Stability-indicating method is an analytical
procedure that is capable of discriminating between
the major active (intact) pharmaceutical ingredients
(API) from any degradation (decomposition)
product(s) formed under defined storage conditions
during stability evaluation period. Forced degradation
studies are indespensible in development of stability
indicating and degradant monitoring method as part
of validation protocol. Chromatographic factor should
be evaluated to optimized of the stability-indicating
of HPLC method for detection for all potentially
relevant degradant. An appropriate sample solvent
and mobile phase must be found that afford suitability
and compatibility with the component of interest as
well as impurities and degradants.
REFERENCE
[1] Patel RM, Shri B. M shaha collage of
pharmaceutical education and research,
modasa, Gujrat, India. stability indicating
HPLC method development a riview,
(international research journal of pharmacy,
2011; 2(5): 79-87.
[2] Shah BP, Jain S, Prajapati KK, Mansuri NY,
stabilityindicating HPLC method development:
a review, International journal of
pharmaceutical science and research
[3] Varsha Rao B, G, Naga Sowjanya, Ajitha A,
Umamaheshwara Rao V, Department of
pharmaceutical analysis and Quality assurance
CMR college of pharmacy. volume 4(08)405-
423.
[4] Saudagar R. B, Manisha mahale, Stability
indicating HPLC method development: a
review, Journal of drug delivery and therapeutic
2019; 9(3-s): 1103-1104.
[5] FDA guidelines of industry, practical
procedures and methods validation (draft
guideline), August 2000.
[6] Monika bakshi and saranjit Singh,
Development of validated stability indicating
assay method a critical review, Pharm. biomed.
Anal. 2002 ; 28(6) 1011-1040.
[7] John v. Dolan Stability indicating assay LC
troubleshooting LCGC North America, 2002;
20(4), 346-349.
[8] Michael J, Smela, Regulatory consideration of
stability indicating Analytical Method in Drug
Substance and Drug Product Testing, American
pharmaceutical review. 2005; 8(3). 51-54
[9] Monika Bakshi and Saranjit Singh:
Development of validated stability-indicating
assay methods--critical review. J. Pharm.
Biomed. Anal. 2002; 28(6): 1011-1040
[10] JohnW. Dolan: Stability-Indicating Assays. LC
Troubleshooting. LCGC North America, 2002;
20(4): 346-349.
[11] Donald D, Hong and Mumtaz Shah,
Development and Validation of HPLC stability
indicating assay, In; Sen T Carstensan, C. T
Rhodes. Editors Drug stability and Principle](https://image.slidesharecdn.com/38stabilityindicatinghplcmethoddevelopmentareview-220226121500/95/Stability-Indicating-HPLC-Method-Development-A-Review-7-638.jpg)
![International Journal of Trend in Scientific Research and Development @ www.ijtsrd.com eISSN: 2456-6470
@ IJTSRD | Unique Paper ID – IJTSRD46342 | Volume – 5 | Issue – 6 | Sep-Oct 2021 Page 257
and Practice. 3rd
edition, New York Marcel
Dekker Inc. 2008; p, 132.
[12] John V. Dolan, Stability-Indicating Assay”
Troubleshooting problems 2005, 275.
[13] Seble Wegaw, Johnson tredrow, Tim grieme,
Lalit Bavda, Wiefeng wang, Shekhar
Vishwanath, et al, HPLC Guide
htpp://www.chemgroups.northwastern,Edu/Sch
eldt/ PDFs.
[14] The united states pharmacopeia, USP 28-NF
23, <1225>, 2005.
[15] LR Snyder, JL Glitch, JJ Kirkland: Practical
HPLC method Development. New York: John
Wiley; 1988; 227-251
[16] J. Li and P. W. Carr: Anal. Chem. 69; 1997:
837–847
[17] Polite L, Liquid chromatography: basic
overview. In: Miller J, Crowther JB [ends],
Analytical chemistry in a GMP environment: a
practical guide. John Wiley & sons, New York,
2000.
[18] Ruan J, Tattersall P, Lozano R, Shah P. The
role of forced degradation studies in stability
indicating HPLC method development. A M.
Pharm Rev. 2006; 9: 46-53.
[19] GA Shabir. Validation of HPLC
Chromatography Methods for Pharmaceutical
Analysis. Understanding the Differences and
Similarities between Validation Requirements
of FDA, the US Pharmacopeia and the ICH. J.
Chromatogr. A. 2003; 987(1-2): 57-66.
[20] Xiao KP, Xiong Y, Liu FZ, Rustum AM.
Efficient method development strategy for
challenging separations of pharmaceutical
molecules using advanced chromatographic
technologies. J Chromatogram A. 2007; 1163:
145-156.
[21] International Conference on Harmonization of
Technical Requirements for Registration of
Pharmaceuticals for Human Use (ICH), Quality
Guidelines, http: //www. ich. org/
products/guidelines/quality/ article/ quality-
guidelines. html.
[22] Stepensky D, Chorny M, Dabour Z,
Schumacher I. Long-term stability study of L
adrenaline injections: Kinetics of sulfonation
and racemization pathways of drug
degradation. Pharm Sci. 2004; 93: 969-980.
[23] ICH Guidance for Industry, Q1B: Photo
stability Testing of New Drug Substances and
Product, International Conference on
Harmonization. Available from:
〈http://www.fda.gov/downloads/Drugs/Guidanc
eComplianceRegulatoryInformation/Guidance’
s/ucm073373.pdf〉, 1996.
[24] D. W. Reynolds, K. L. Facchine, J. F.
Mullaney, etal., Available guidance and best
practices for conducting forced degradation
studies, Pharm. Technol. 2002; 26(2): 48–56.
[25] H. Brummer, how to approach a forced
degradation study, Life Sci. Technol. Bull.
2011; 31: 1–4.
[26] ICH guidelines Q1A (R2). Stability Testing of
New Drug Substances and Products (revision
2), November 2003.
[27] Reynolds DW, Facchine KL, Mullaney JF,
Alsante KM, Hatajik TD, Motto MG: Available
guidance and best practices for conducting
forced degradation studies. Pharm Tech; 2002:
48-56.
[28] Kalpesh N Patel, Jayvadan K. Patel, Ganesh C.
Rajput, Naresh B. Rajgor; Derivative
spectrometry method for chemical analysis: A
review; Der Pharmacia Lettre, 2010, 2(2): 139-
150.
[29] Tiwari G, Tiwari R, Srivastava B, Rai A,
Pathak K (2008) Simultaneous estimation of
Metronidazole and Amoxicillin in synthetic
mixture by ultraviolet spectroscopy. AJRC;
1(2): 91-94](https://image.slidesharecdn.com/38stabilityindicatinghplcmethoddevelopmentareview-220226121500/95/Stability-Indicating-HPLC-Method-Development-A-Review-8-638.jpg)