Embed presentation
Download to read offline








The document outlines key interview preparation questions related to Clinical Data Management (CDM), including definitions, importance, phases, and common tools used. CDM ensures data quality and integrity through structured processes, consisting of setup, conduct, and close-out phases which include essential activities such as data entry and protocol adherence. Additionally, it emphasizes the critical role of a protocol in guiding the research process and maintaining the integrity of data collection and analysis.











Overview of the presentation, focusing on key Clinical Data Management (CDM) interview preparation content.
Index outlining the key topics related to CDM, including definitions, importance, tools, phases, and protocols.
CDM is defined as a process for collecting, validating, and archiving clinical data under regulatory standards.
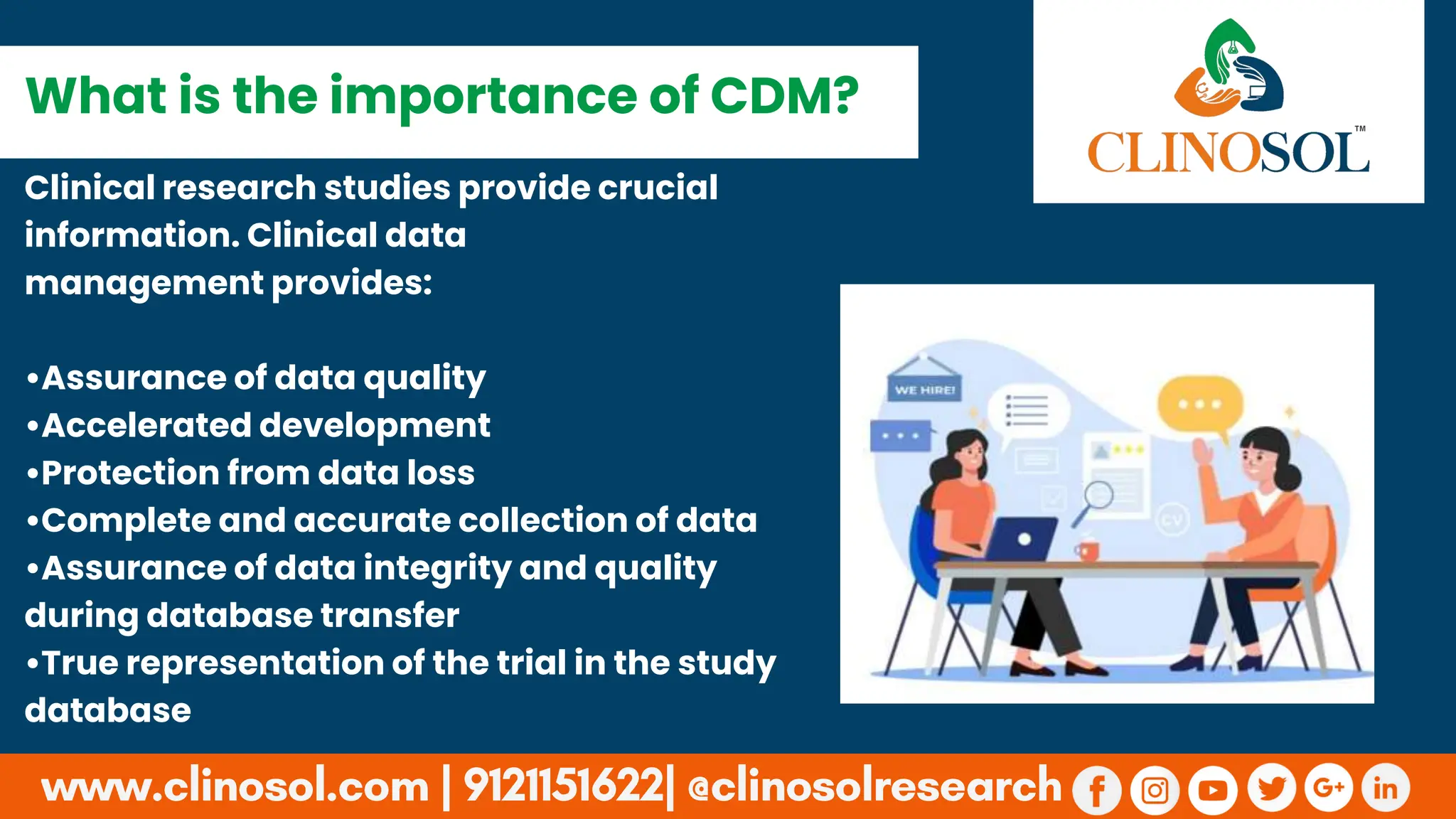
Highlights the significance of CDM in ensuring data quality, integrity, and accelerated clinical development.
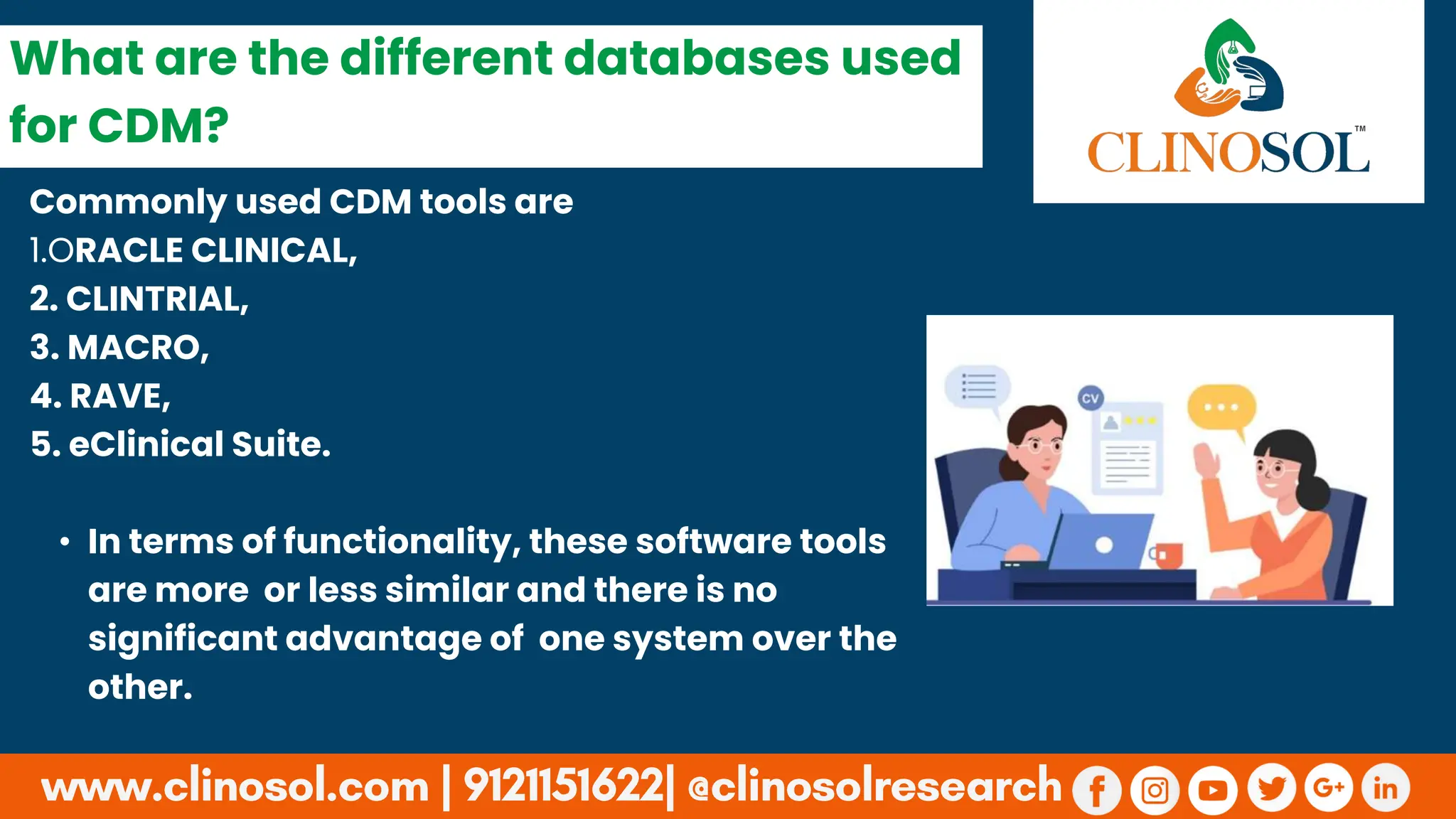
Discussion of commonly used CDM tools such as Oracle Clinical and ClinTrial, noting their similar functionalities.
Outlines the three phases of CDM: setup, conduct, and close-out, detailing various activities in each phase.
Explains the role of a protocol as a guiding document in clinical research, detailing its importance in study design and data collection.
Brief mention of achievements in 2023, suggesting progress or milestones reached in CDM.