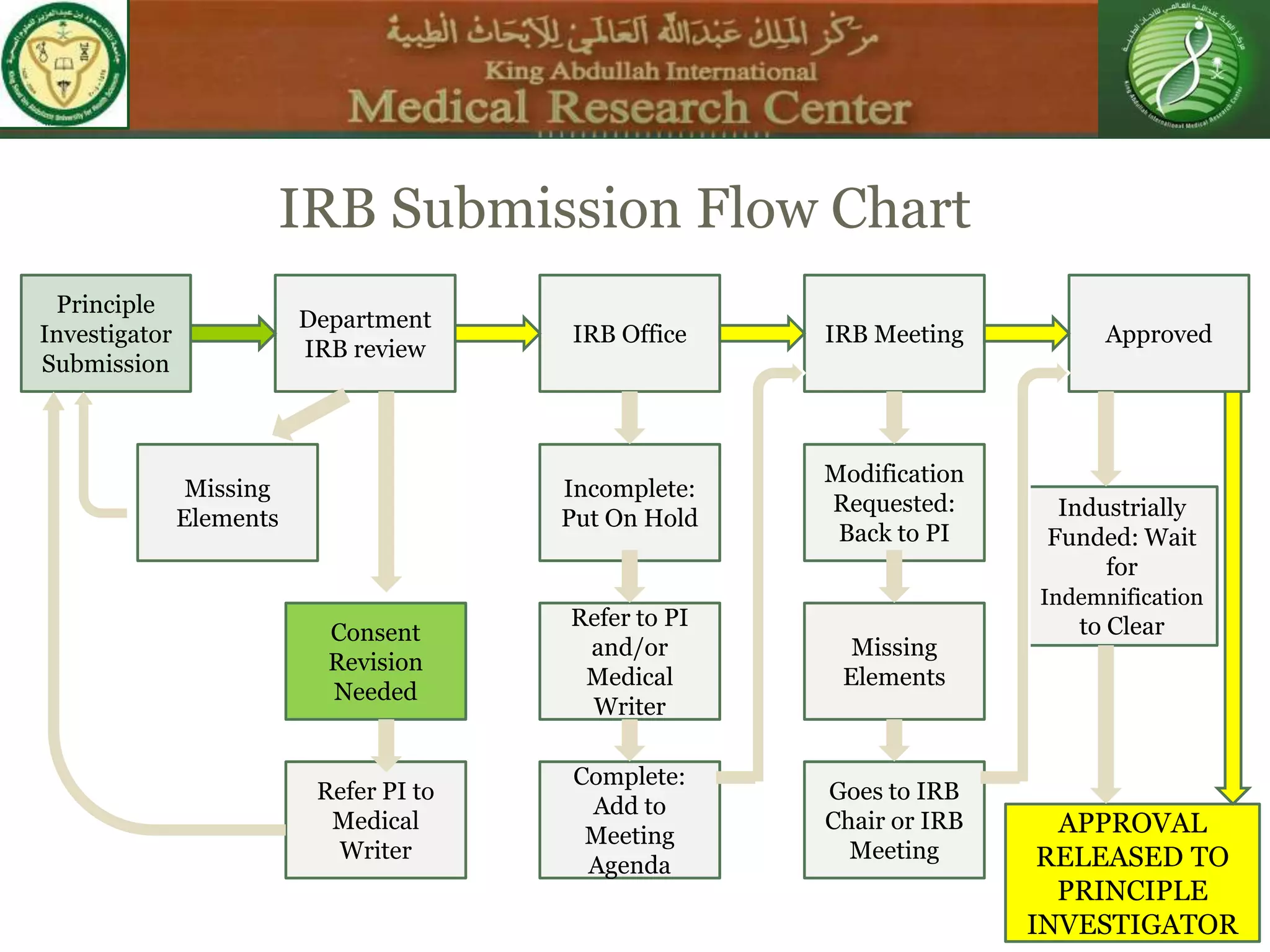
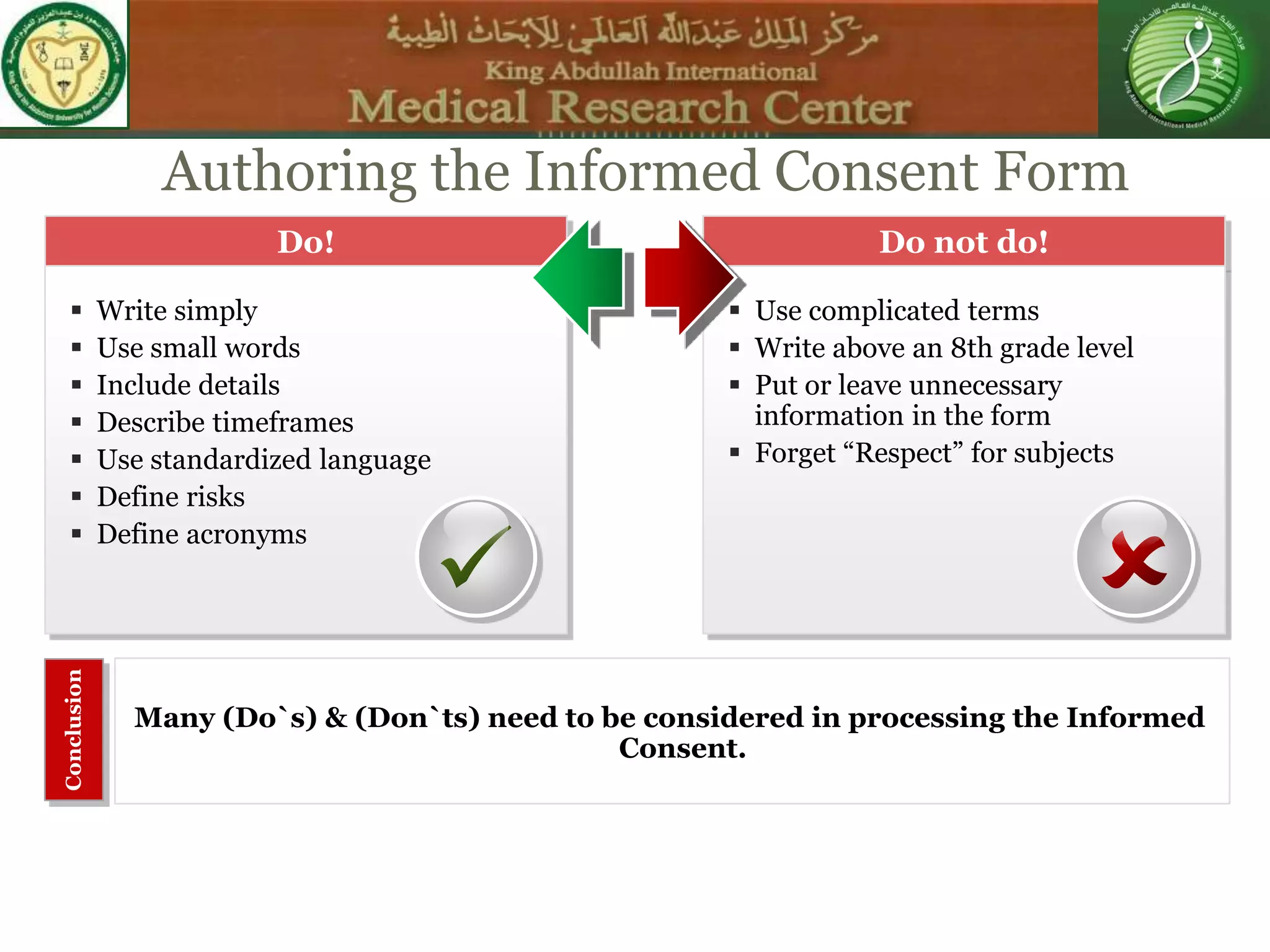
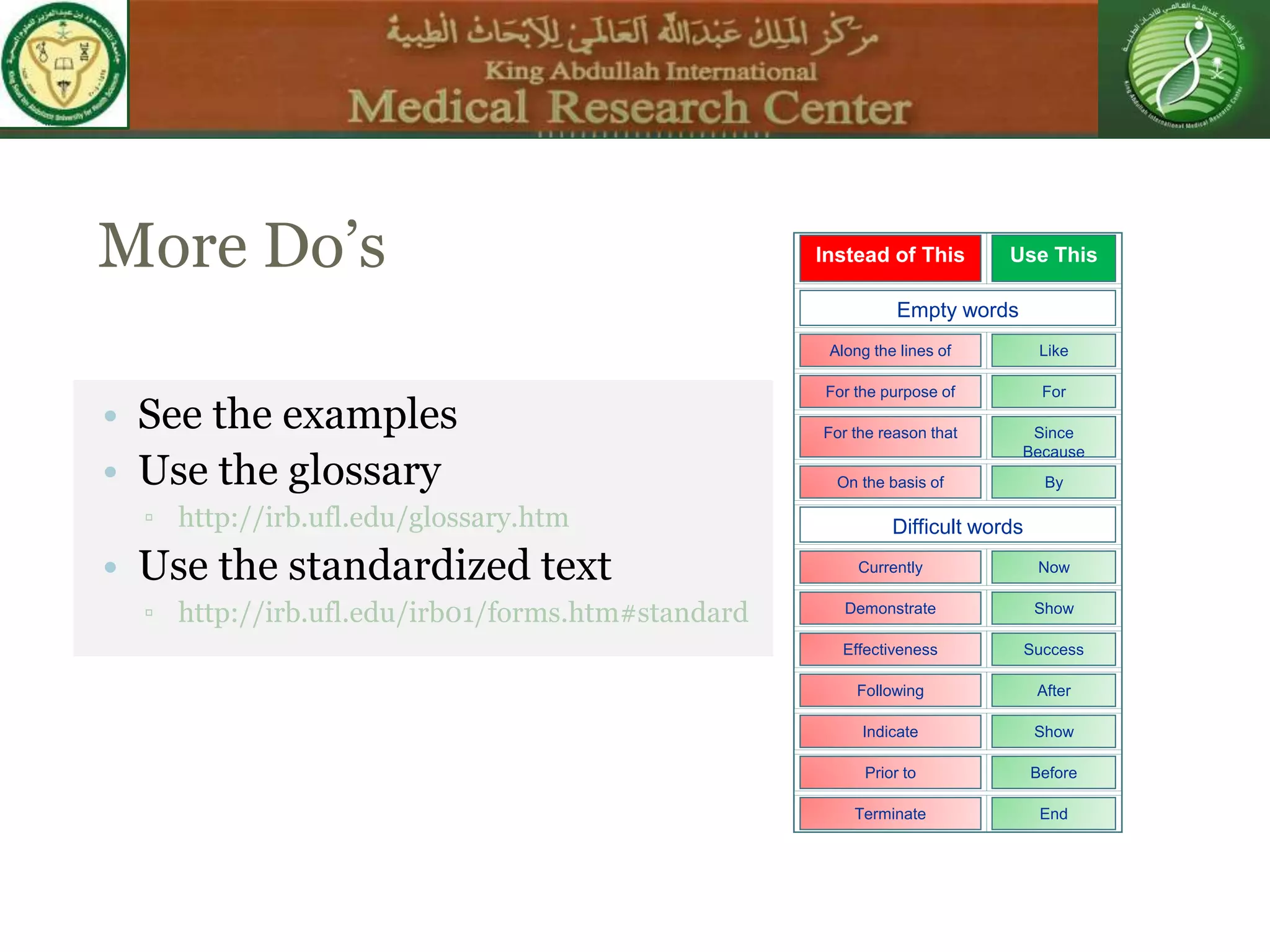
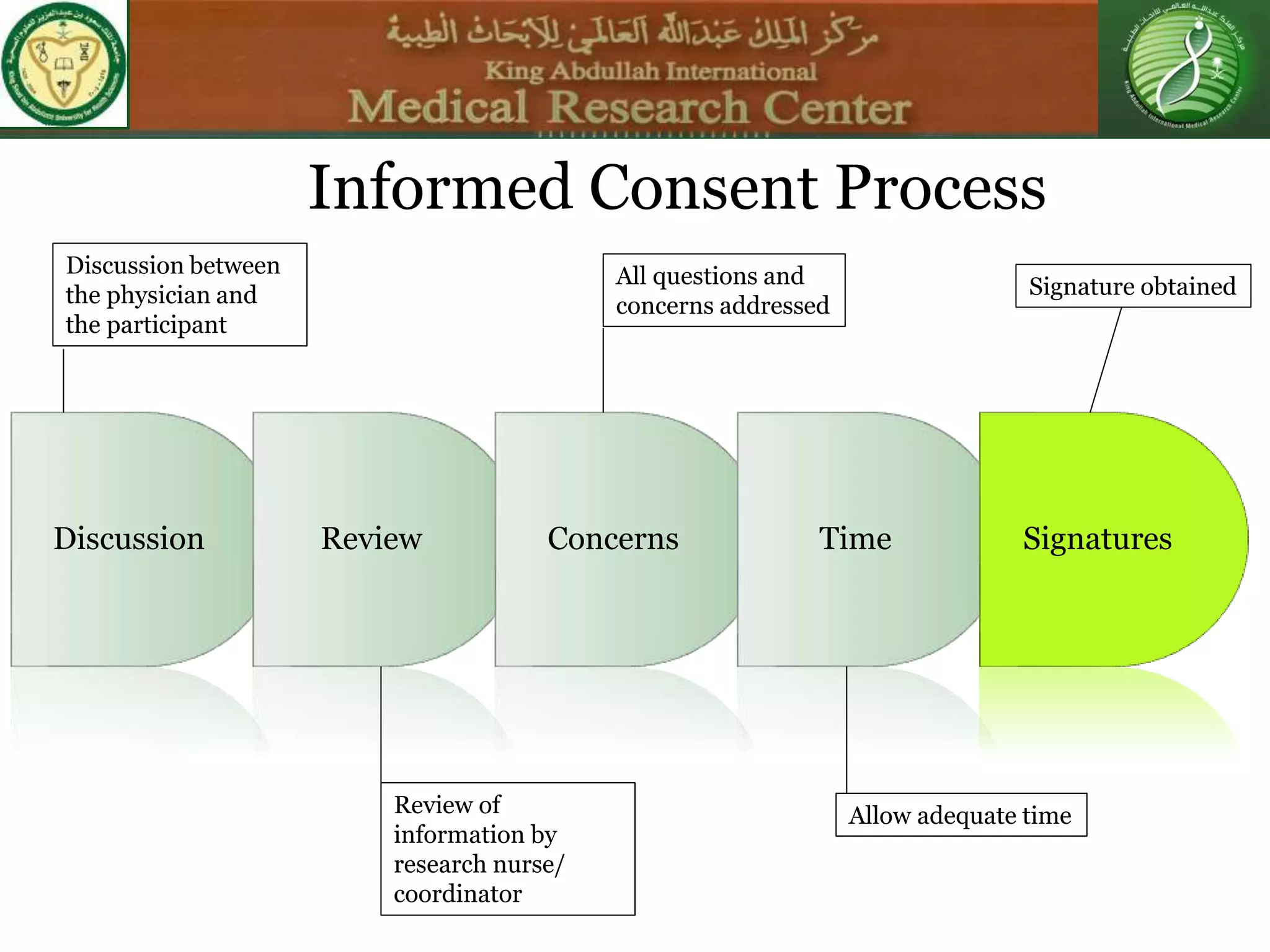
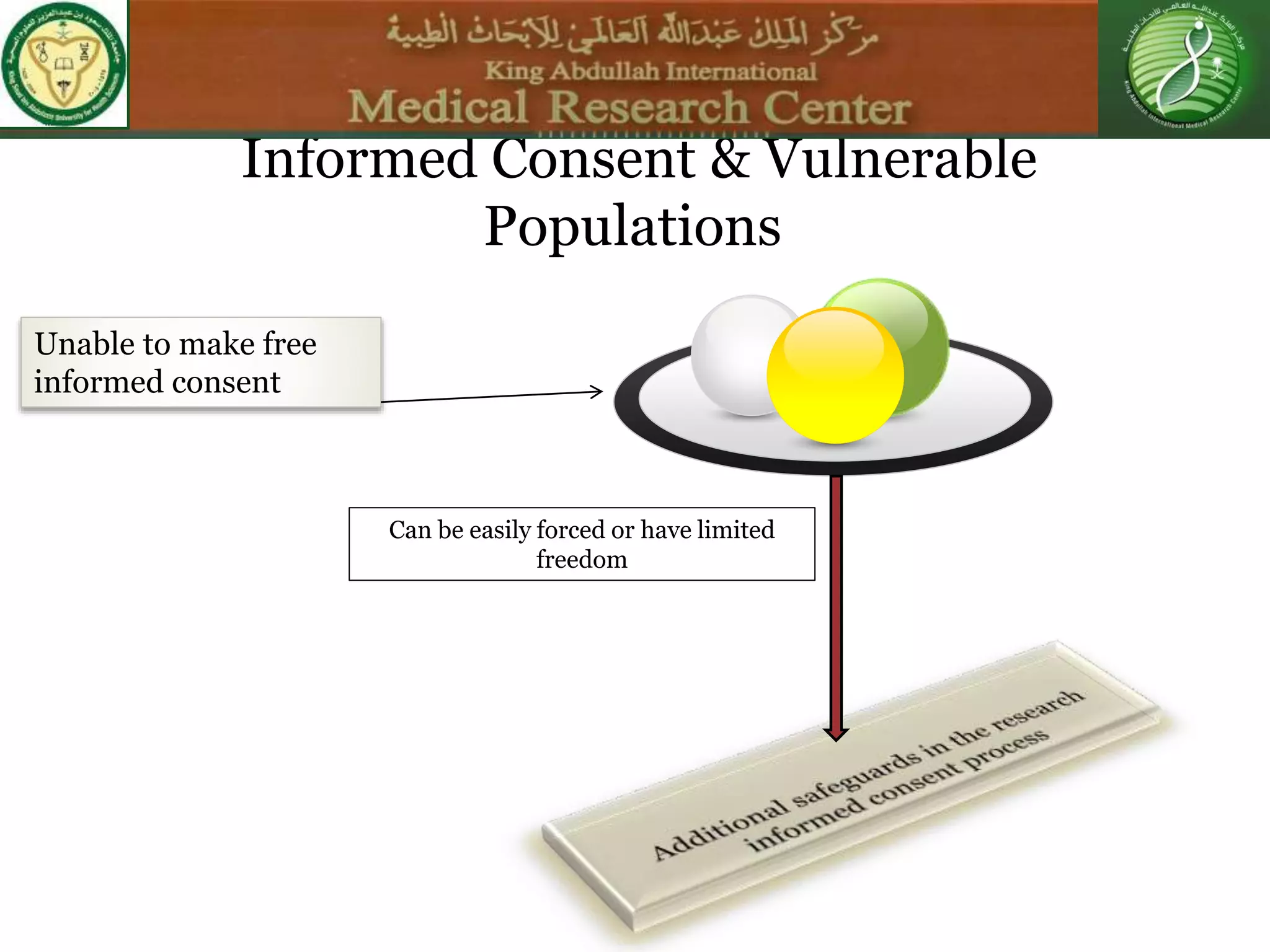
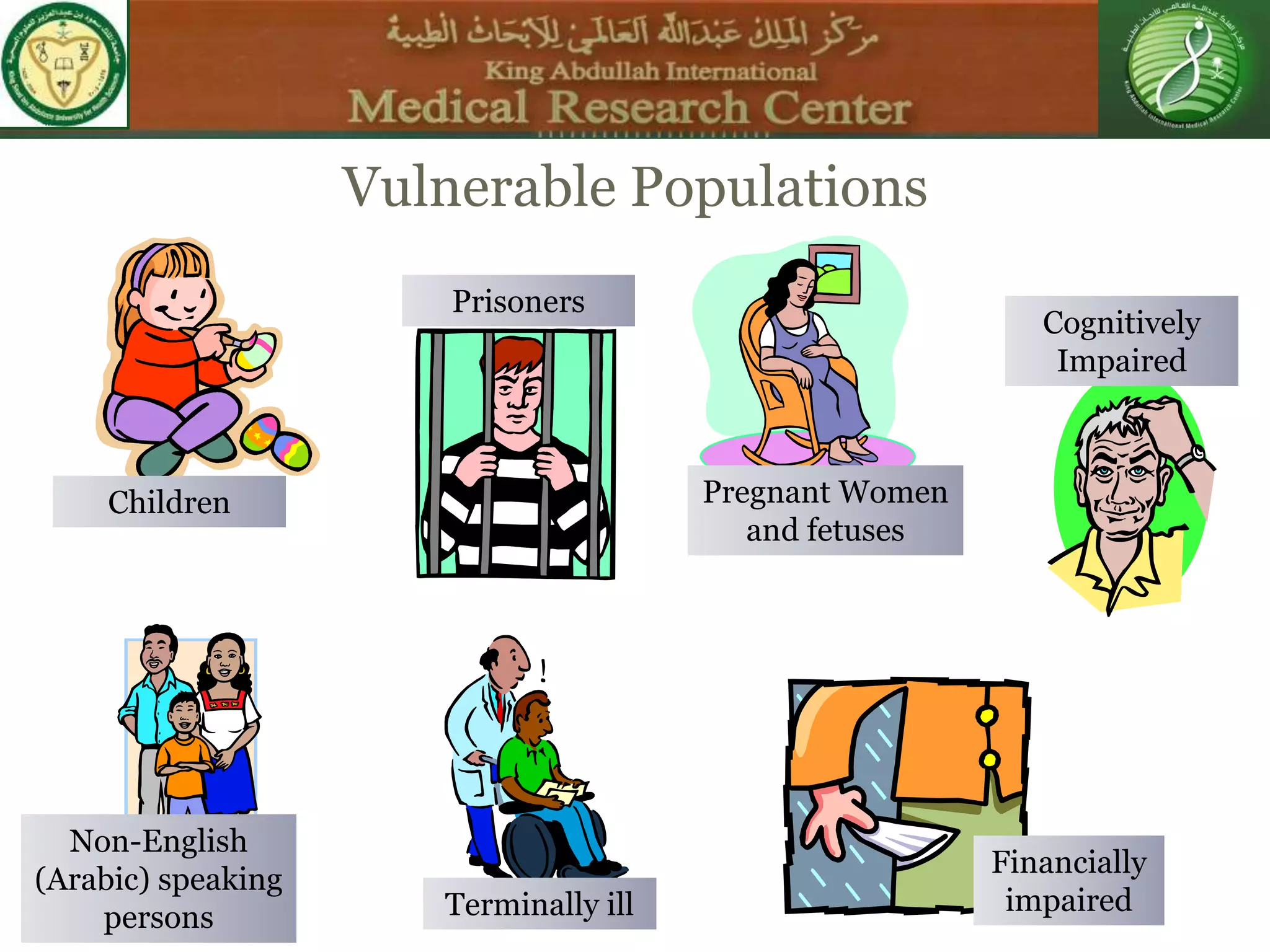
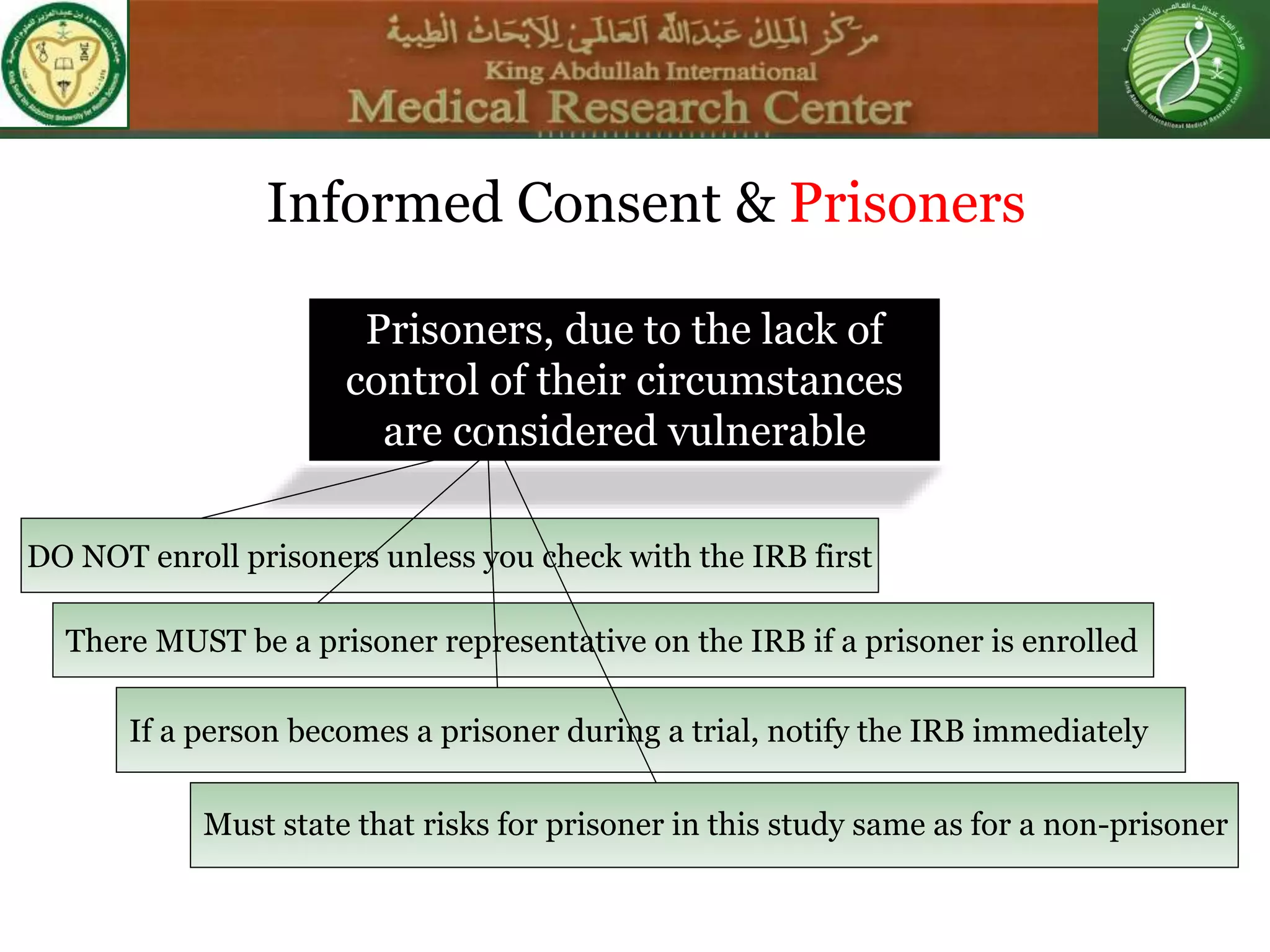
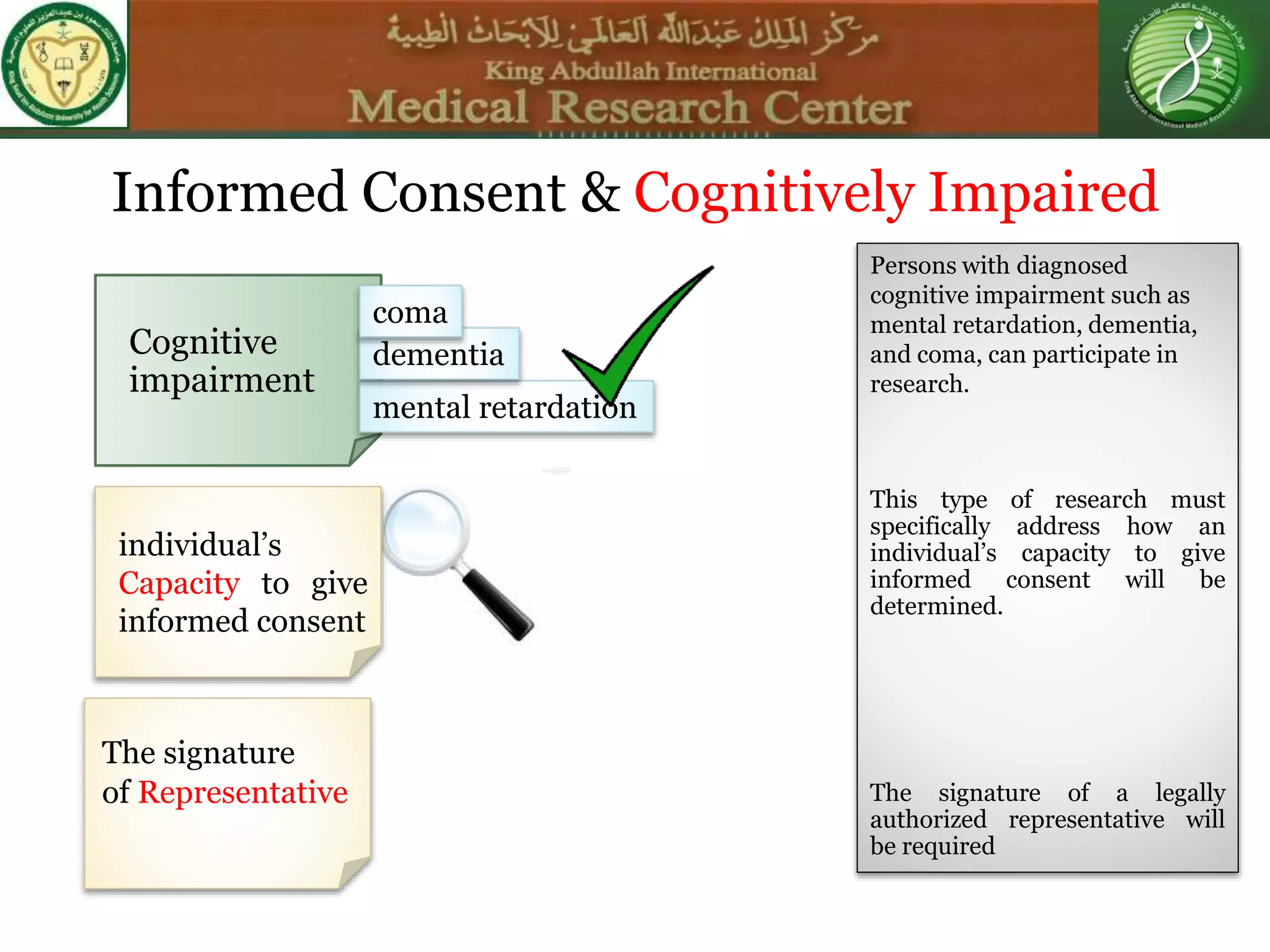
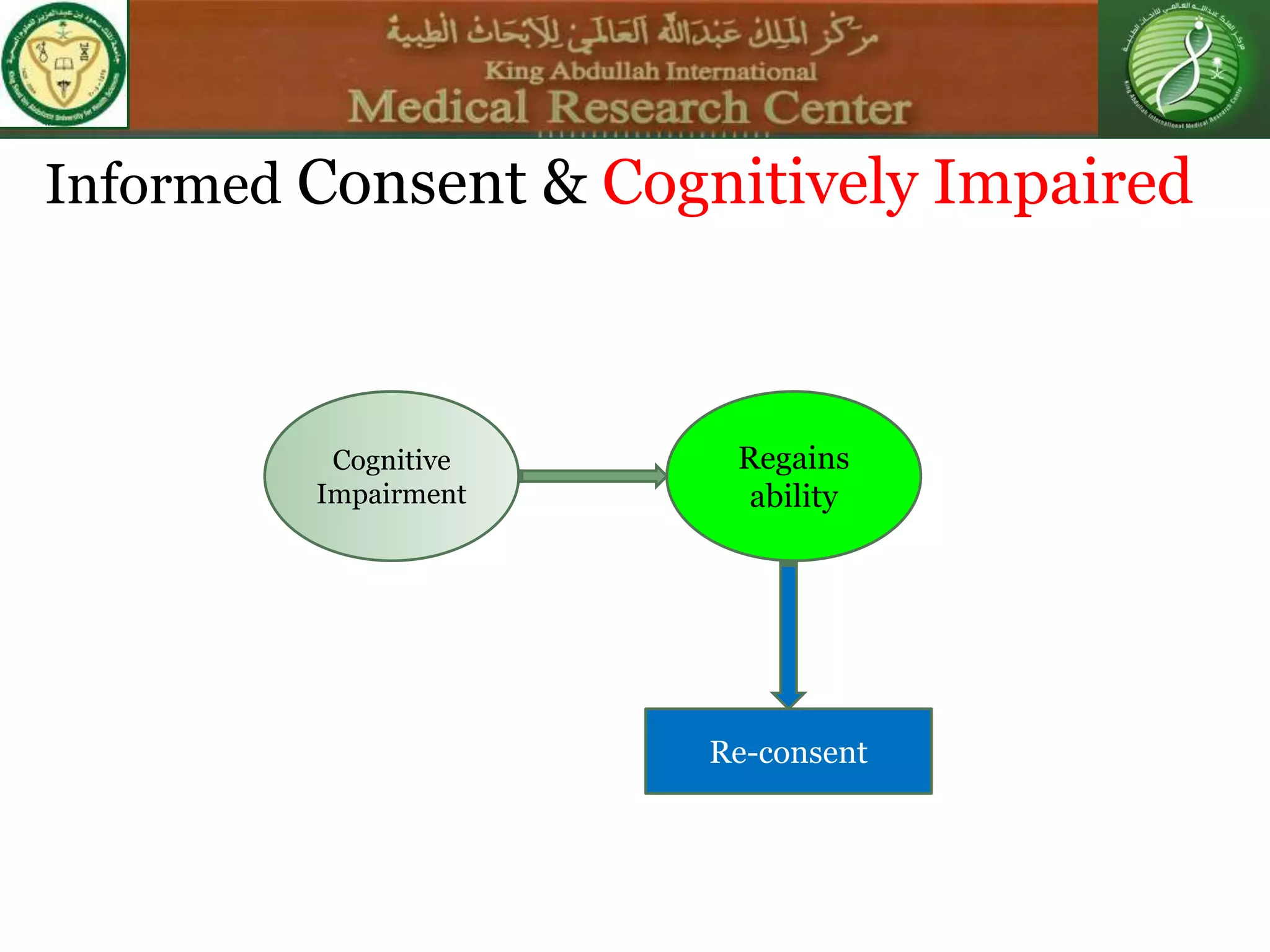
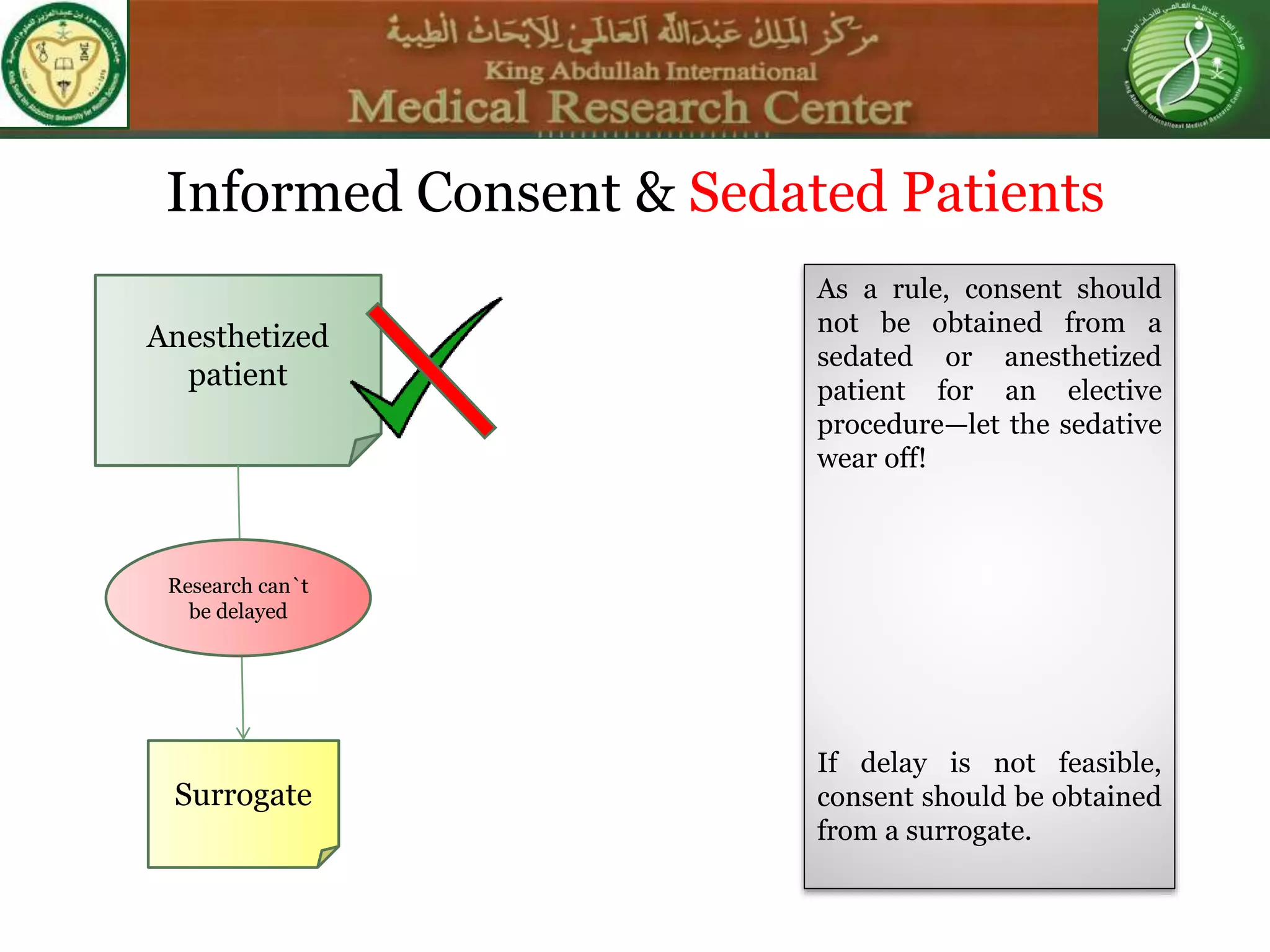
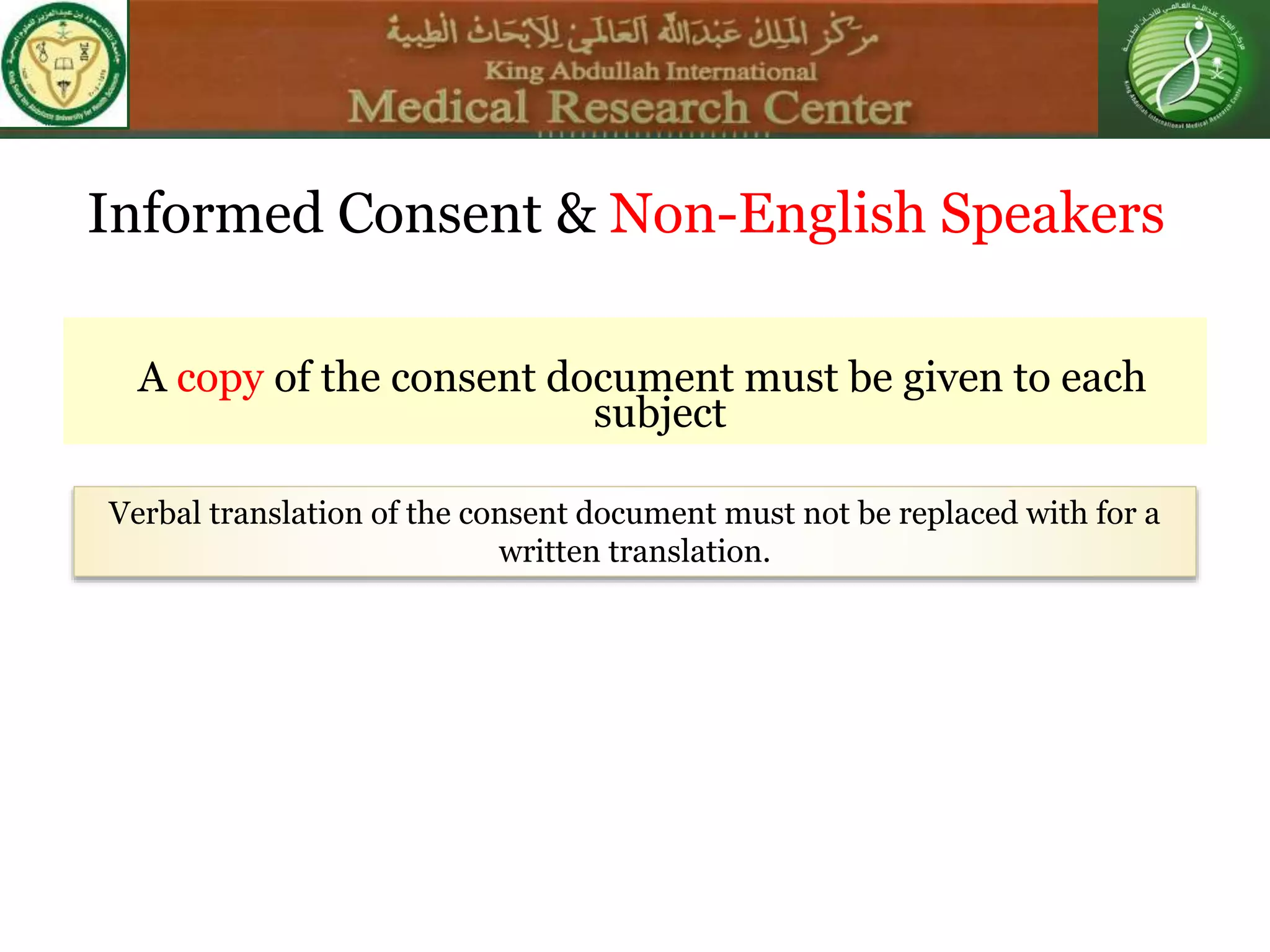
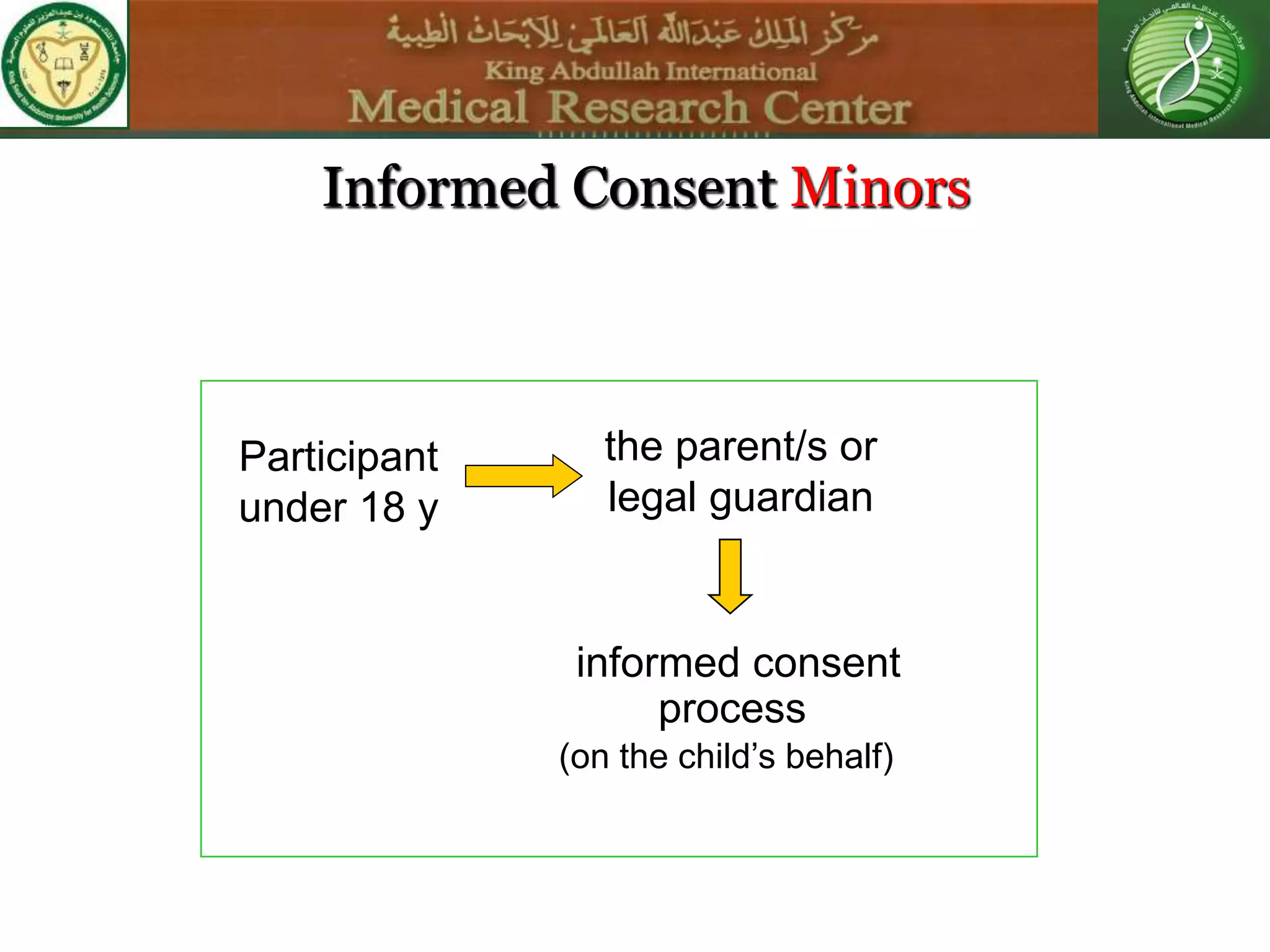
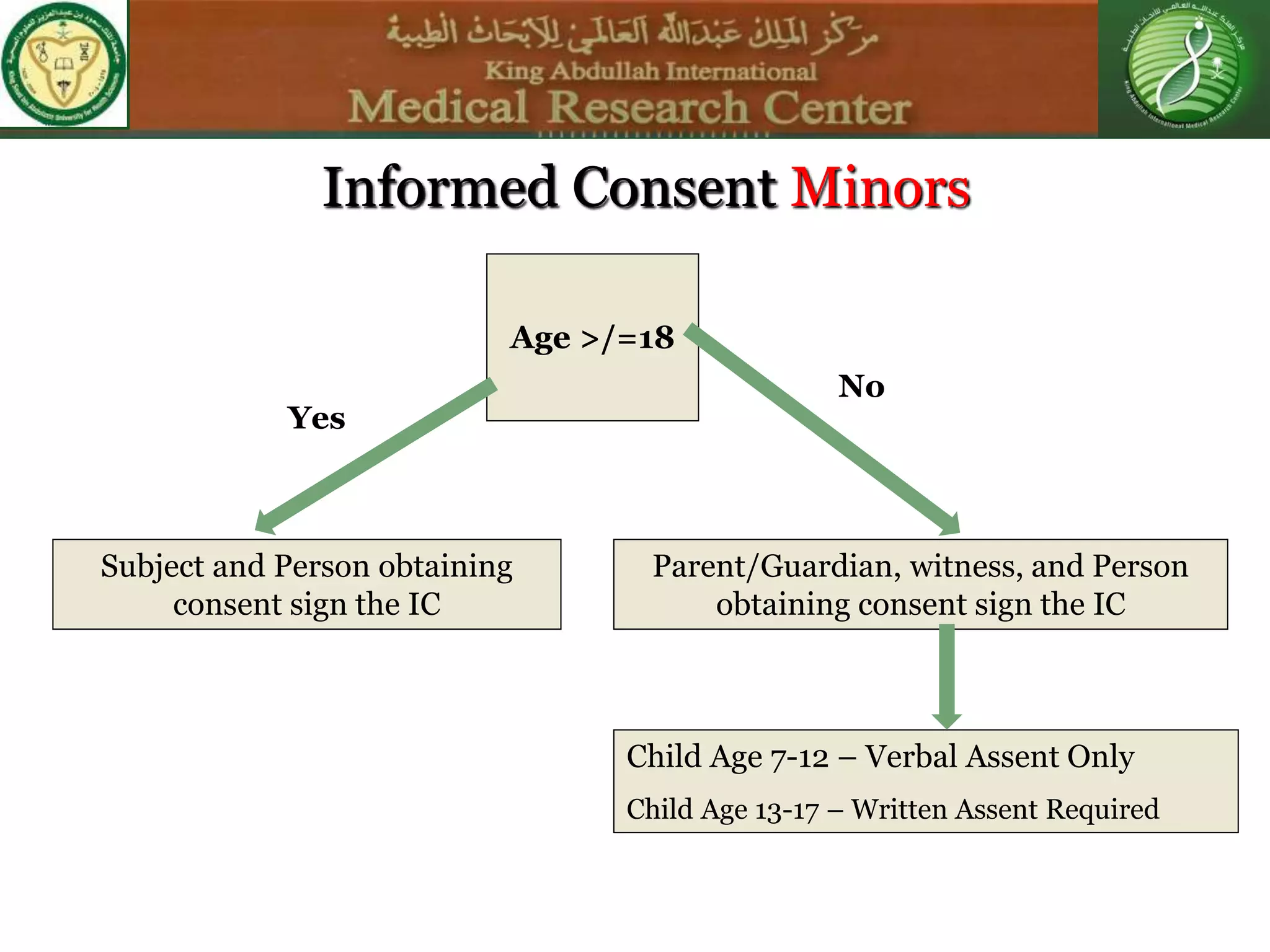
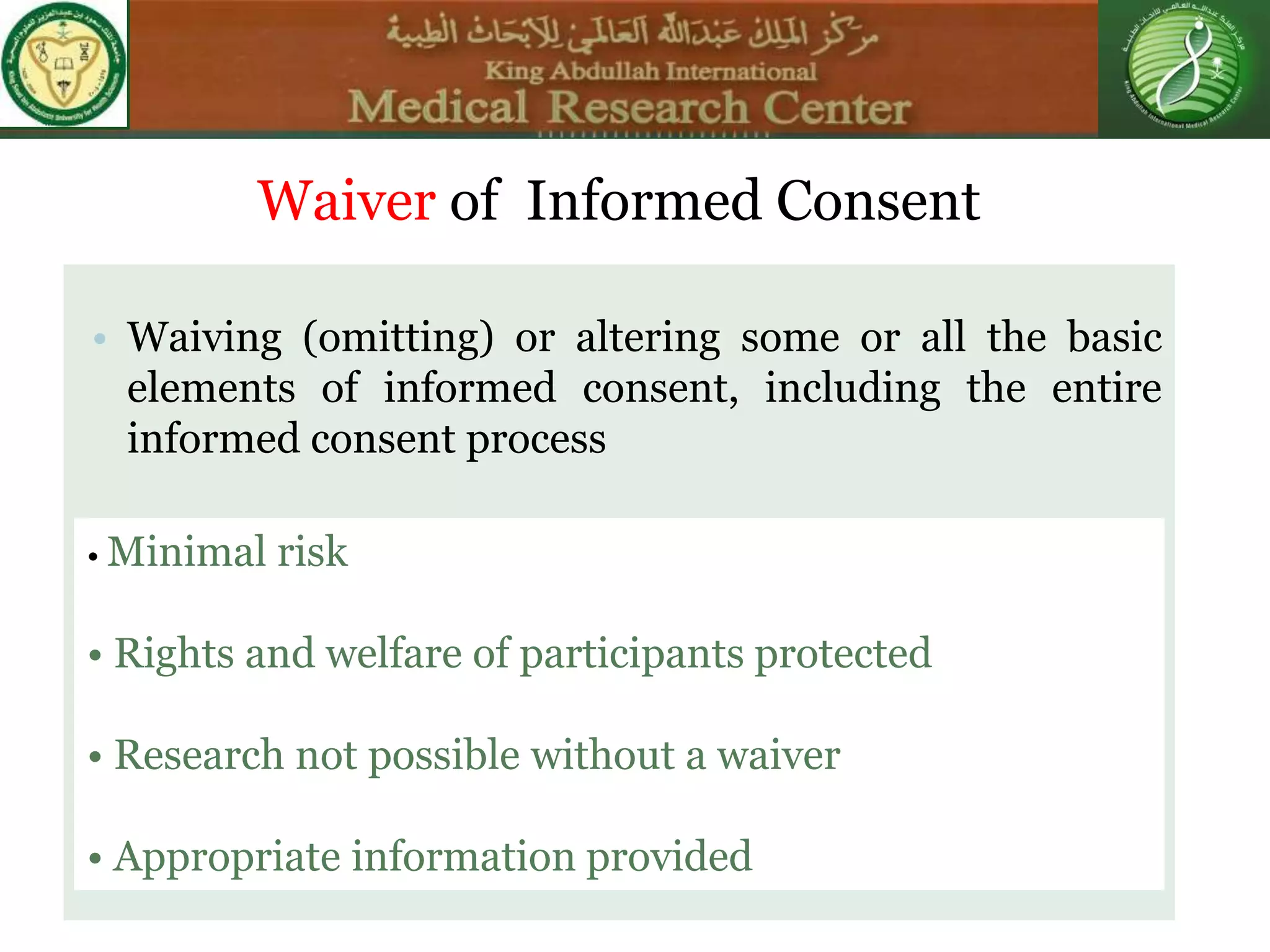
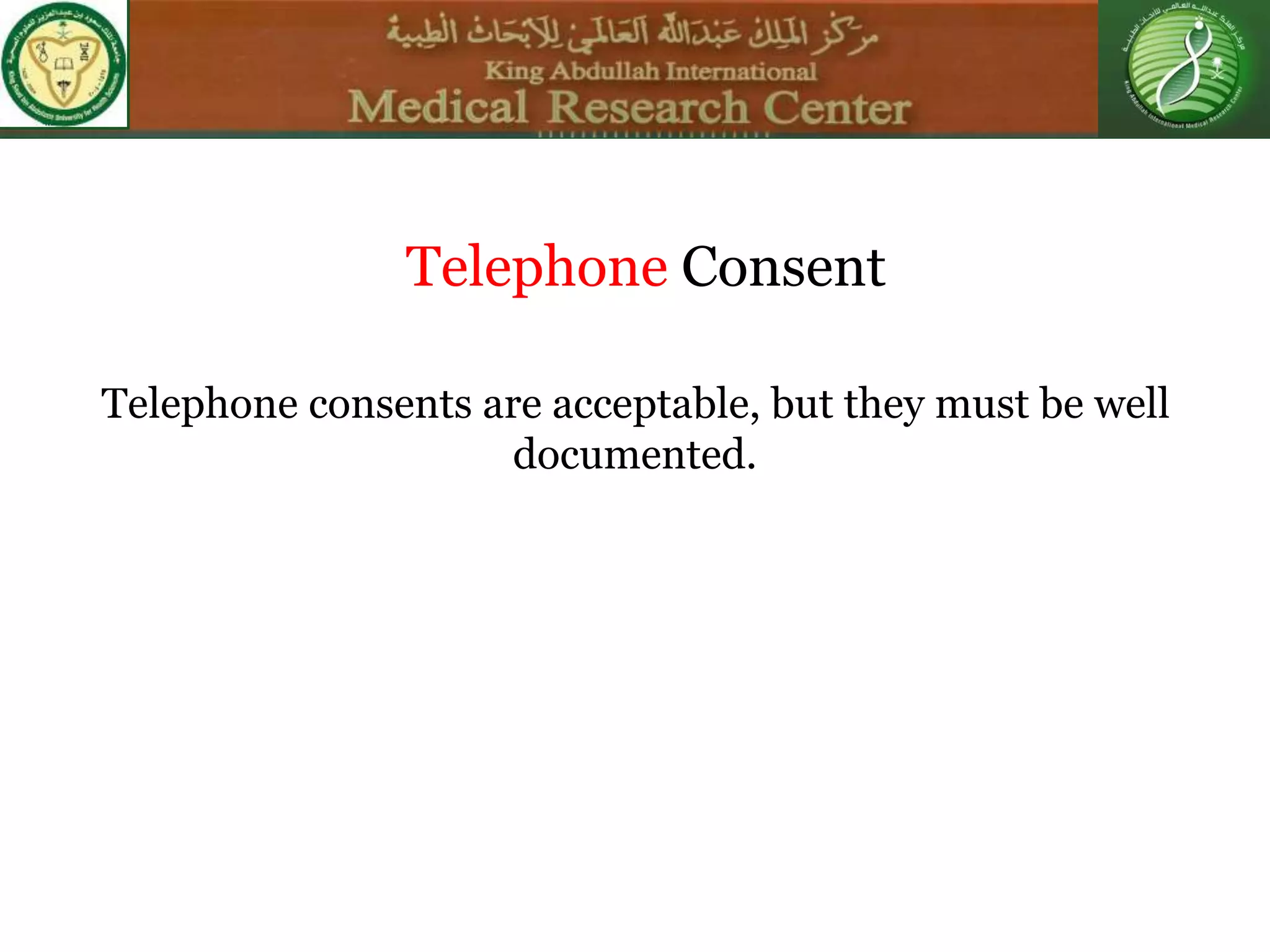
This document discusses the informed consent process. It begins by explaining that informed consent is a process, not just a form. It then provides an IRB submission flow chart and discusses the rules and guidelines that must be followed for informed consent. The document outlines best practices for authoring informed consent forms, including using plain language and avoiding medical jargon. It discusses informed consent considerations for vulnerable populations like children, prisoners, non-English speakers, and cognitively impaired individuals. The document also covers topics like obtaining consent from legally authorized representatives, waiving or altering elements of informed consent, revoking consent, and responsibilities at the end of a study.