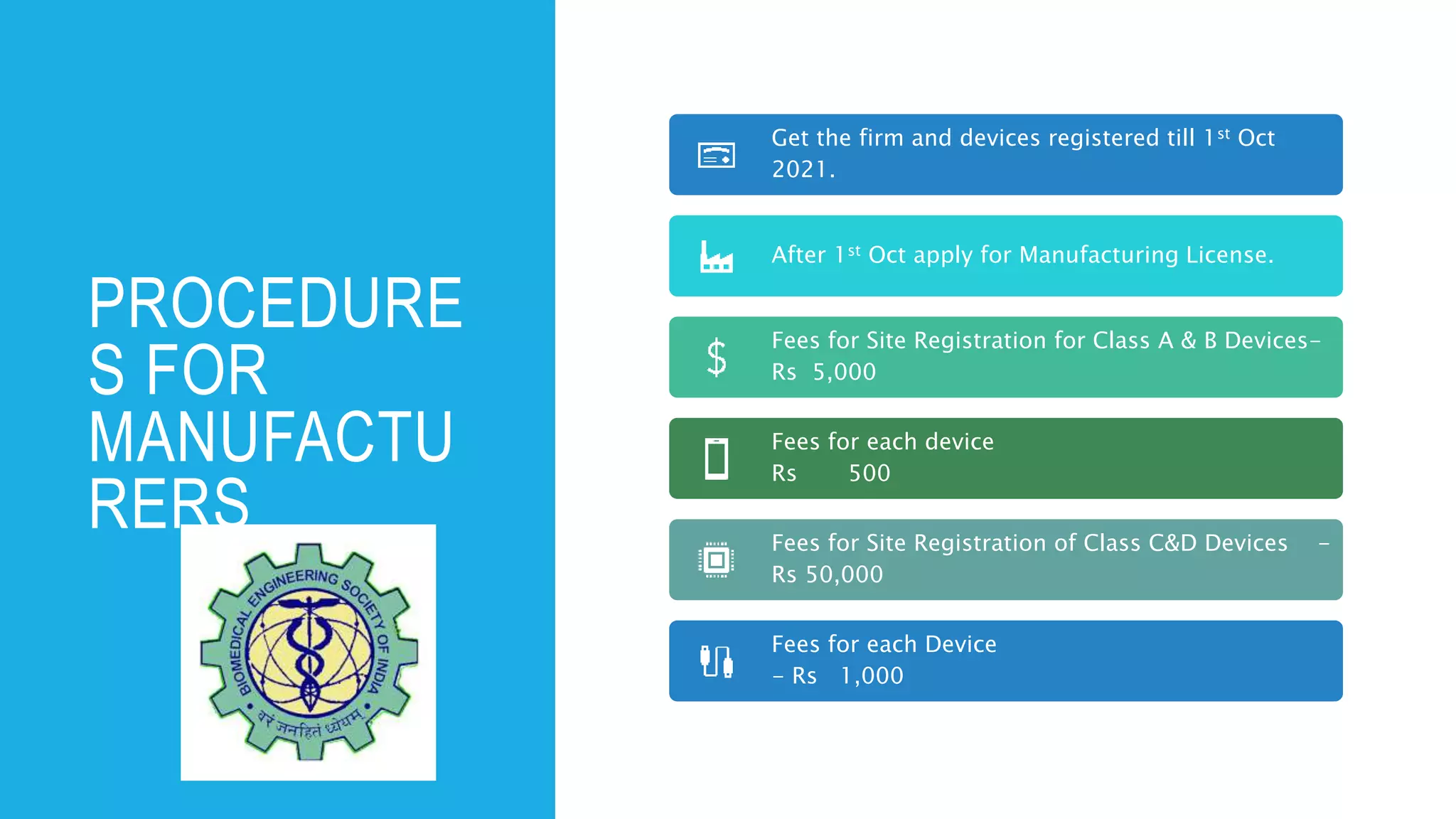
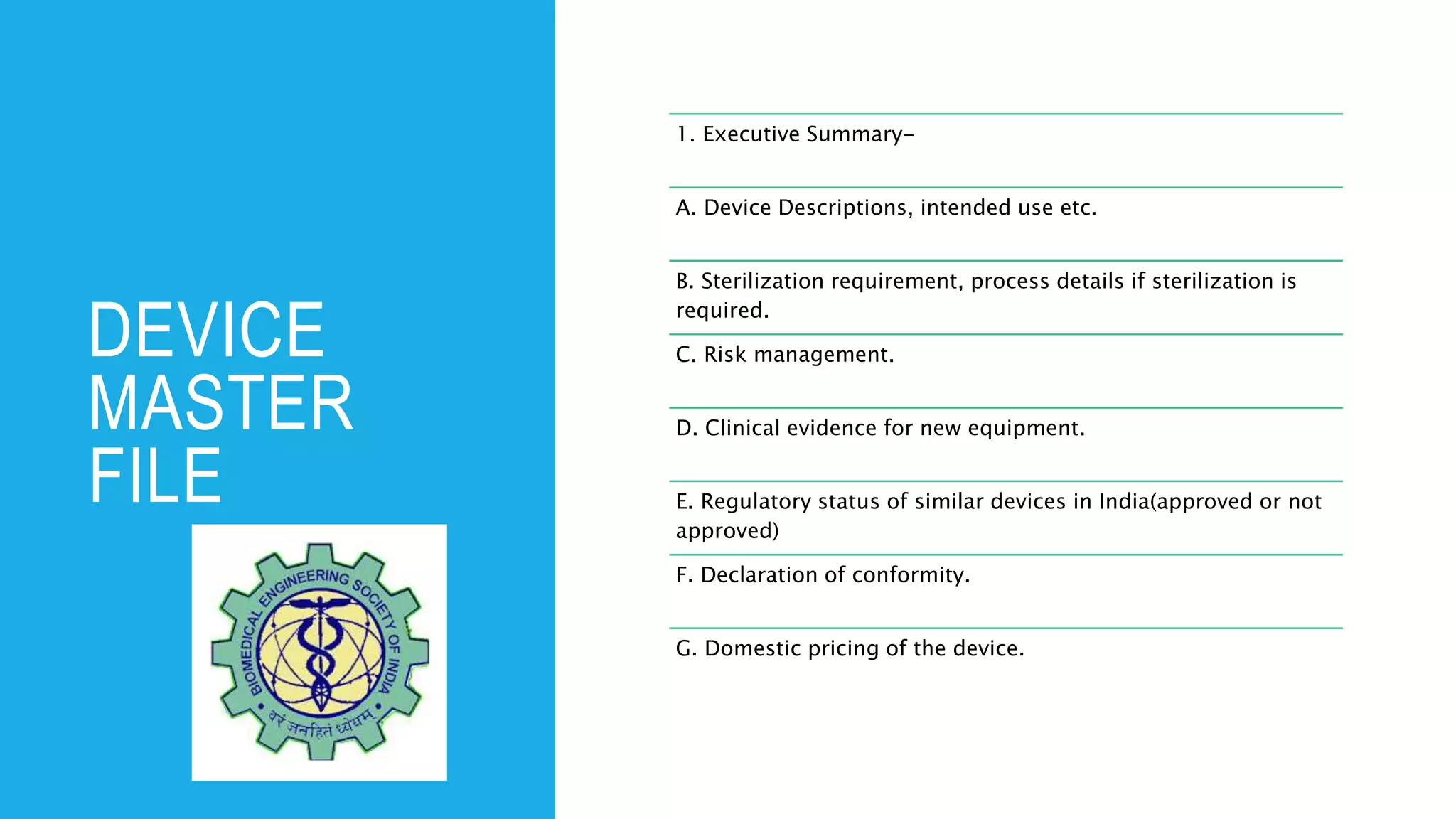
The document outlines the implementation of the Medical Devices Rules 2017 in India, emphasizing the phases for registration and licensing of medical devices to ensure compliance and safety. It details timelines for mandatory registration and licensing for different classes of medical devices, along with penalties for non-compliance. Additionally, it provides guidelines for manufacturers and importers regarding documentation and processes for obtaining necessary licenses.