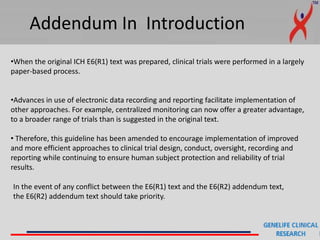
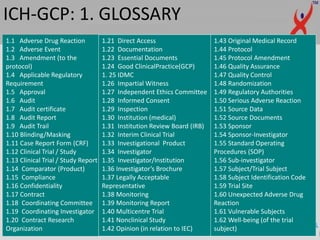
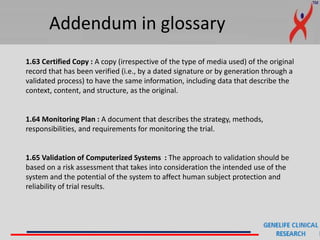
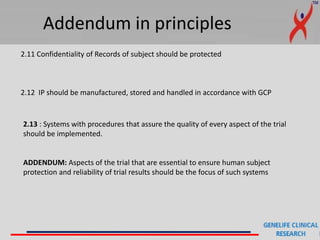
This document provides a summary of guidelines for good clinical practice (GCP) according to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). It discusses the purpose and scope of GCP, which is to ensure proper design, conduct, and reporting of clinical trials involving human subjects. Key topics covered include ethics review, responsibilities of investigators and sponsors, informed consent of subjects, clinical trial documentation and record keeping. The document emphasizes protecting the rights, safety and well-being of clinical trial subjects.