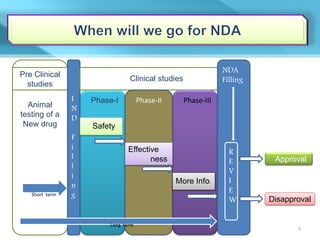
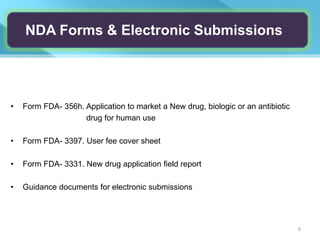
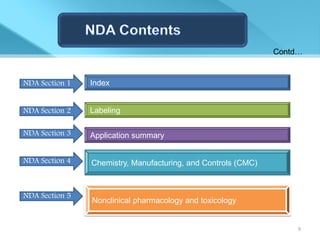
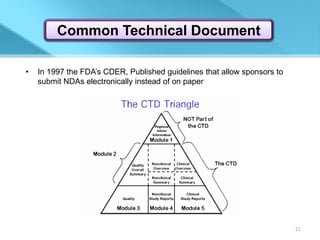
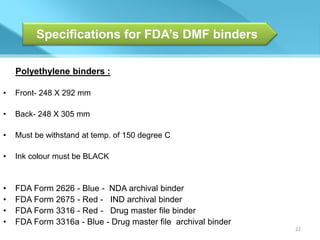
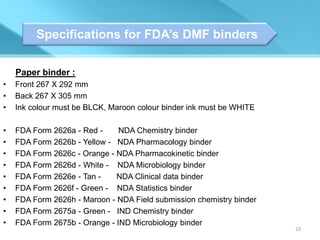
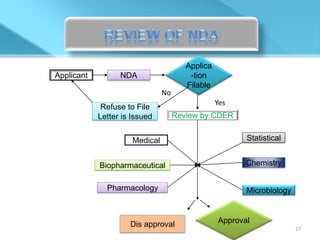
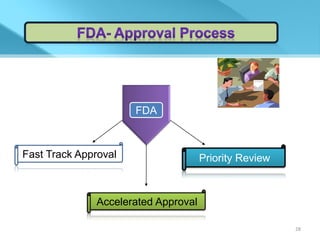
This document provides an overview of Vineeth Kumar Ekbote's lab presentation on new drug applications (NDAs). The presentation covers what an NDA is, the goals and process of an NDA, the forms and contents required in an NDA submission, guidance documents for NDAs, and how NDAs are reviewed and approved by the FDA. The presentation also describes the various sections required in an NDA, including the application summary, chemistry and manufacturing controls, clinical data, and labeling.